当前位置:
X-MOL 学术
›
Org. Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
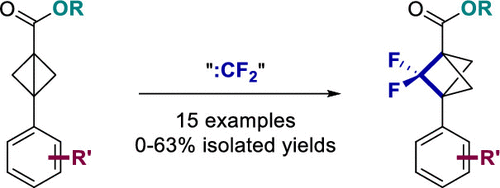
A Selective Synthesis of 2,2-Difluorobicyclo[1.1.1]pentane Analogues: “BCP-F2”
Organic Letters ( IF 4.9 ) Pub Date : 2019-07-11 , DOI: 10.1021/acs.orglett.9b02026 Xiaoshen Ma 1 , David L. Sloman 1 , Yongxin Han 1 , David J. Bennett 1
Organic Letters ( IF 4.9 ) Pub Date : 2019-07-11 , DOI: 10.1021/acs.orglett.9b02026 Xiaoshen Ma 1 , David L. Sloman 1 , Yongxin Han 1 , David J. Bennett 1
Affiliation
|
The bicyclo[1.1.1]pentane (BCP) motif has been utilized as bioisosteres in drug candidates to replace phenyl, tert-butyl, and alkynyl fragments in order to improve physicochemical properties. However, bceause of the difficulty of synthesis, most BCP analogues prepared only bear 1,3-“para”-substituents. We report the first selective synthesis of 2,2-difluorobicyclo[1.1.1]pentanes via difluorocarbene insertion into bicyclo[1.1.0]butanes. Moreover, this methodology should inspire future studies on synthesis of other “ortho/meta-substituted” BCPs via similar mechanisms.
中文翻译:

2,2-二氟双环[1.1.1]戊烷类似物的选择性合成:“ BCP-F2”
双环[1.1.1]戊烷(BCP)基序已被用作候选药物中的生物等排体,以取代苯基,叔丁基和炔基片段,以改善其理化性质。然而,由于合成的困难,大多数制备的BCP类似物仅带有1,3-“对”取代基。我们报告通过二氟卡宾插入双环[1.1.0]丁烷中的第一个选择性合成2,2-二氟双环[1.1.1]戊烷。此外,这种方法应能激发通过类似机制合成其他“邻位/间位取代的” BCP的未来研究。
更新日期:2019-07-12
中文翻译:

2,2-二氟双环[1.1.1]戊烷类似物的选择性合成:“ BCP-F2”
双环[1.1.1]戊烷(BCP)基序已被用作候选药物中的生物等排体,以取代苯基,叔丁基和炔基片段,以改善其理化性质。然而,由于合成的困难,大多数制备的BCP类似物仅带有1,3-“对”取代基。我们报告通过二氟卡宾插入双环[1.1.0]丁烷中的第一个选择性合成2,2-二氟双环[1.1.1]戊烷。此外,这种方法应能激发通过类似机制合成其他“邻位/间位取代的” BCP的未来研究。







































 京公网安备 11010802027423号
京公网安备 11010802027423号