当前位置:
X-MOL 学术
›
Chem. Soc. Rev.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Intercalation chemistry of graphite: alkali metal ions and beyond
Chemical Society Reviews ( IF 40.4 ) Pub Date : 2019-07-11 00:00:00 , DOI: 10.1039/c9cs00162j Yuqi Li 1, 2, 3, 4, 5 , Yaxiang Lu 1, 2, 3, 4, 5 , Philipp Adelhelm 6, 7, 8, 9 , Maria-Magdalena Titirici 10, 11, 12, 13, 14 , Yong-Sheng Hu 1, 2, 3, 4, 5
Chemical Society Reviews ( IF 40.4 ) Pub Date : 2019-07-11 00:00:00 , DOI: 10.1039/c9cs00162j Yuqi Li 1, 2, 3, 4, 5 , Yaxiang Lu 1, 2, 3, 4, 5 , Philipp Adelhelm 6, 7, 8, 9 , Maria-Magdalena Titirici 10, 11, 12, 13, 14 , Yong-Sheng Hu 1, 2, 3, 4, 5
Affiliation
|
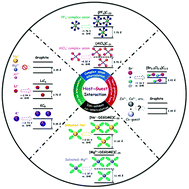
Reversibly intercalating ions into host materials for electrochemical energy storage is the essence of the working principle of rocking-chair type batteries. The most relevant example is the graphite anode for rechargeable Li-ion batteries which has been commercialized in 1991 and still represents the benchmark anode in Li-ion batteries 30 years later. Learning from past lessons on alkali metal intercalation in graphite, recent breakthroughs in sodium and potassium intercalation in graphite have been demonstrated for Na-ion batteries and K-ion batteries. Interestingly, some significant differences proved to exist for the intercalation of Na+ and K+ into graphite compared with the Li+ case. Such different host–guest interactions are unique depending on the host materials and electrolytes, which greatly contribute to a deeper understanding of intercalation-type electrode materials for next generation alkali metal ion batteries. This review summarizes significant advances from both experimental and theoretical calculations with a focus on comparing the intercalation of three alkali metal ions (Li+, Na+, K+) into graphite and aims to clarify the intimate host–guest relationships and the underlying mechanisms. New approaches developed to achieve favorable intercalation coupled with the challenges in this field are also discussed. We also extrapolate alkali metal ion intercalation in graphite to mono-/multi-valent ions in layered electrode materials, which will deepen the understanding of intercalation chemistry and provide guidance to explore new guests and hosts.
中文翻译:

石墨的插层化学:碱金属离子及其他
可逆地将离子插入主体材料中以进行电化学能量存储是摇椅型电池工作原理的本质。最相关的例子是可充电锂离子电池的石墨阳极,该阳极已于1991年商业化,并且在30年后仍代表着锂离子电池的基准阳极。从过去关于石墨中碱金属嵌入的经验教训中吸取教训,已证明钠离子电池和K离子电池在石墨中钠和钾的嵌入方面取得了最新突破。有趣的是,事实证明,与Li +相比,Na +和K +嵌入石墨中存在一些显着差异。案子。取决于主体材料和电解质,这种不同的主体-客体相互作用是独特的,这极大地有助于更深入地了解下一代碱金属离子电池的插层型电极材料。这篇综述总结了实验和理论计算的重要进展,重点是比较三种碱金属离子(Li +,Na +,K +)转化为石墨,旨在阐明亲密的宾客关系及其潜在机制。还讨论了为实现良好插层而开发的新方法以及该领域中的挑战。我们还将石墨中的碱金属离子嵌入推断为层状电极材料中的单价/多价离子,这将加深对嵌入化学的理解,并为探索新的来宾和宿主提供指导。
更新日期:2019-07-11
中文翻译:

石墨的插层化学:碱金属离子及其他
可逆地将离子插入主体材料中以进行电化学能量存储是摇椅型电池工作原理的本质。最相关的例子是可充电锂离子电池的石墨阳极,该阳极已于1991年商业化,并且在30年后仍代表着锂离子电池的基准阳极。从过去关于石墨中碱金属嵌入的经验教训中吸取教训,已证明钠离子电池和K离子电池在石墨中钠和钾的嵌入方面取得了最新突破。有趣的是,事实证明,与Li +相比,Na +和K +嵌入石墨中存在一些显着差异。案子。取决于主体材料和电解质,这种不同的主体-客体相互作用是独特的,这极大地有助于更深入地了解下一代碱金属离子电池的插层型电极材料。这篇综述总结了实验和理论计算的重要进展,重点是比较三种碱金属离子(Li +,Na +,K +)转化为石墨,旨在阐明亲密的宾客关系及其潜在机制。还讨论了为实现良好插层而开发的新方法以及该领域中的挑战。我们还将石墨中的碱金属离子嵌入推断为层状电极材料中的单价/多价离子,这将加深对嵌入化学的理解,并为探索新的来宾和宿主提供指导。













































 京公网安备 11010802027423号
京公网安备 11010802027423号