当前位置:
X-MOL 学术
›
Cell Metab.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Oncogene Amplification in Growth Factor Signaling Pathways Renders Cancers Dependent on Membrane Lipid Remodeling.
Cell Metabolism ( IF 27.7 ) Pub Date : 2019-07-11 , DOI: 10.1016/j.cmet.2019.06.014
Junfeng Bi 1 , Taka-Aki Ichu 2 , Ciro Zanca 1 , Huijun Yang 1 , Wei Zhang 3 , Yuchao Gu 4 , Sudhir Chowdhry 1 , Alex Reed 2 , Shiro Ikegami 5 , Kristen M Turner 1 , Wenjing Zhang 1 , Genaro R Villa 4 , Sihan Wu 1 , Oswald Quehenberger 6 , William H Yong 7 , Harley I Kornblum 8 , Jeremy N Rich 9 , Timothy F Cloughesy 7 , Webster K Cavenee 10 , Frank B Furnari 11 , Benjamin F Cravatt 2 , Paul S Mischel 11
Cell Metabolism ( IF 27.7 ) Pub Date : 2019-07-11 , DOI: 10.1016/j.cmet.2019.06.014
Junfeng Bi 1 , Taka-Aki Ichu 2 , Ciro Zanca 1 , Huijun Yang 1 , Wei Zhang 3 , Yuchao Gu 4 , Sudhir Chowdhry 1 , Alex Reed 2 , Shiro Ikegami 5 , Kristen M Turner 1 , Wenjing Zhang 1 , Genaro R Villa 4 , Sihan Wu 1 , Oswald Quehenberger 6 , William H Yong 7 , Harley I Kornblum 8 , Jeremy N Rich 9 , Timothy F Cloughesy 7 , Webster K Cavenee 10 , Frank B Furnari 11 , Benjamin F Cravatt 2 , Paul S Mischel 11
Affiliation
|
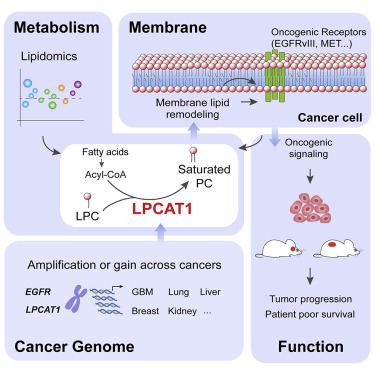
Advances in DNA sequencing technologies have reshaped our understanding of the molecular basis of cancer, providing a precise genomic view of tumors. Complementary biochemical and biophysical perspectives of cancer point toward profound shifts in nutrient uptake and utilization that propel tumor growth and major changes in the structure of the plasma membrane of tumor cells. The molecular mechanisms that bridge these fundamental aspects of tumor biology remain poorly understood. Here, we show that the lysophosphatidylcholine acyltransferase LPCAT1 functionally links specific genetic alterations in cancer with aberrant metabolism and plasma membrane remodeling to drive tumor growth. Growth factor receptor-driven cancers are found to depend on LPCAT1 to shape plasma membrane composition through enhanced saturated phosphatidylcholine content that is, in turn, required for the transduction of oncogenic signals. These results point to a genotype-informed strategy that prioritizes lipid remodeling pathways as therapeutic targets for diverse cancers.
中文翻译:

生长因子信号通路中的癌基因扩增导致依赖于膜脂质重塑的癌症。
DNA测序技术的进步重塑了我们对癌症分子基础的理解,从而提供了精确的肿瘤基因组视图。癌症的互补生化和生物物理观点指向营养吸收和利用的深刻变化,从而推动肿瘤生长和肿瘤细胞质膜结构的重大变化。桥接肿瘤生物学这些基本方面的分子机制仍然知之甚少。在这里,我们表明,溶血磷脂酰胆碱酰基转移酶LPCAT1功能性地连接癌症中的特定基因改变与异常代谢和质膜重塑,以驱动肿瘤的生长。发现生长因子受体驱动的癌症依赖LPCAT1通过增强的饱和磷脂酰胆碱含量来塑造质膜组成,即,反过来,这是致癌信号转导所必需的。这些结果指出了基因型信息化策略,该策略优先考虑脂质重塑途径作为多种癌症的治疗靶标。
更新日期:2019-09-30
中文翻译:

生长因子信号通路中的癌基因扩增导致依赖于膜脂质重塑的癌症。
DNA测序技术的进步重塑了我们对癌症分子基础的理解,从而提供了精确的肿瘤基因组视图。癌症的互补生化和生物物理观点指向营养吸收和利用的深刻变化,从而推动肿瘤生长和肿瘤细胞质膜结构的重大变化。桥接肿瘤生物学这些基本方面的分子机制仍然知之甚少。在这里,我们表明,溶血磷脂酰胆碱酰基转移酶LPCAT1功能性地连接癌症中的特定基因改变与异常代谢和质膜重塑,以驱动肿瘤的生长。发现生长因子受体驱动的癌症依赖LPCAT1通过增强的饱和磷脂酰胆碱含量来塑造质膜组成,即,反过来,这是致癌信号转导所必需的。这些结果指出了基因型信息化策略,该策略优先考虑脂质重塑途径作为多种癌症的治疗靶标。































 京公网安备 11010802027423号
京公网安备 11010802027423号