当前位置:
X-MOL 学术
›
Cell Stem Cell
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Remodeling of Bone Marrow Hematopoietic Stem Cell Niches Promotes Myeloid Cell Expansion during Premature or Physiological Aging.
Cell Stem Cell ( IF 19.8 ) Pub Date : 2019-07-11 , DOI: 10.1016/j.stem.2019.06.007 Ya-Hsuan Ho 1 , Raquel Del Toro 2 , José Rivera-Torres 2 , Justyna Rak 1 , Claudia Korn 1 , Andrés García-García 3 , David Macías 4 , Cristina González-Gómez 2 , Alberto Del Monte 2 , Monika Wittner 5 , Amie K Waller 1 , Holly R Foster 1 , Carlos López-Otín 6 , Randall S Johnson 4 , Claus Nerlov 7 , Cedric Ghevaert 1 , William Vainchenker 8 , Fawzia Louache 5 , Vicente Andrés 2 , Simón Méndez-Ferrer 3
Cell Stem Cell ( IF 19.8 ) Pub Date : 2019-07-11 , DOI: 10.1016/j.stem.2019.06.007 Ya-Hsuan Ho 1 , Raquel Del Toro 2 , José Rivera-Torres 2 , Justyna Rak 1 , Claudia Korn 1 , Andrés García-García 3 , David Macías 4 , Cristina González-Gómez 2 , Alberto Del Monte 2 , Monika Wittner 5 , Amie K Waller 1 , Holly R Foster 1 , Carlos López-Otín 6 , Randall S Johnson 4 , Claus Nerlov 7 , Cedric Ghevaert 1 , William Vainchenker 8 , Fawzia Louache 5 , Vicente Andrés 2 , Simón Méndez-Ferrer 3
Affiliation
|
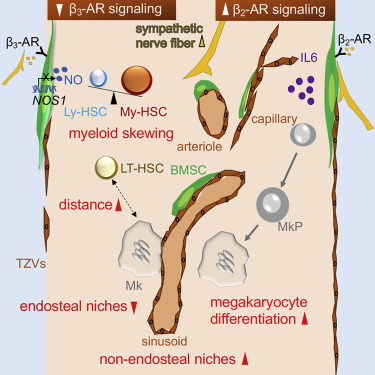
Hematopoietic stem cells (HSCs) residing in the bone marrow (BM) accumulate during aging but are functionally impaired. However, the role of HSC-intrinsic and -extrinsic aging mechanisms remains debated. Megakaryocytes promote quiescence of neighboring HSCs. Nonetheless, whether megakaryocyte-HSC interactions change during pathological/natural aging is unclear. Premature aging in Hutchinson-Gilford progeria syndrome recapitulates physiological aging features, but whether these arise from altered stem or niche cells is unknown. Here, we show that the BM microenvironment promotes myelopoiesis in premature/physiological aging. During physiological aging, HSC-supporting niches decrease near bone but expand further from bone. Increased BM noradrenergic innervation promotes β2-adrenergic-receptor(AR)-interleukin-6-dependent megakaryopoiesis. Reduced β3-AR-Nos1 activity correlates with decreased endosteal niches and megakaryocyte apposition to sinusoids. However, chronic treatment of progeroid mice with β3-AR agonist decreases premature myeloid and HSC expansion and restores the proximal association of HSCs to megakaryocytes. Therefore, normal/premature aging of BM niches promotes myeloid expansion and can be improved by targeting the microenvironment.
中文翻译:

骨髓造血干细胞生态位的重塑促进过早或生理衰老期间的骨髓细胞扩张。
驻留在骨髓(BM)中的造血干细胞(HSC)在衰老过程中积累,但功能受损。然而,HSC 内在和外在衰老机制的作用仍然存在争议。巨核细胞促进邻近 HSC 的静止。尽管如此,巨核细胞-HSC 相互作用是否在病理/自然衰老过程中发生变化尚不清楚。哈钦森-吉尔福德早衰综合症中的过早衰老概括了生理衰老特征,但这些特征是否源于干细胞或生态位细胞的改变尚不清楚。在这里,我们表明骨髓微环境促进过早/生理衰老中的骨髓生成。在生理衰老过程中,HSC 支持的微环境在靠近骨骼的地方减少,但在远离骨骼的地方进一步扩展。 BM 去甲肾上腺素能神经支配的增加促进 β2-肾上腺素能受体 (AR)-白细胞介素 6 依赖性巨核细胞生成。 β3-AR-Nos1 活性降低与骨内膜微环境减少和巨核细胞与血窦并置有关。然而,用 β3-AR 激动剂长期治疗早衰小鼠会减少过早的骨髓和 HSC 扩张,并恢复 HSC 与巨核细胞的近端关联。因此,骨髓微环境的正常/过早衰老会促进骨髓扩张,并且可以通过针对微环境来改善。
更新日期:2019-09-30
中文翻译:

骨髓造血干细胞生态位的重塑促进过早或生理衰老期间的骨髓细胞扩张。
驻留在骨髓(BM)中的造血干细胞(HSC)在衰老过程中积累,但功能受损。然而,HSC 内在和外在衰老机制的作用仍然存在争议。巨核细胞促进邻近 HSC 的静止。尽管如此,巨核细胞-HSC 相互作用是否在病理/自然衰老过程中发生变化尚不清楚。哈钦森-吉尔福德早衰综合症中的过早衰老概括了生理衰老特征,但这些特征是否源于干细胞或生态位细胞的改变尚不清楚。在这里,我们表明骨髓微环境促进过早/生理衰老中的骨髓生成。在生理衰老过程中,HSC 支持的微环境在靠近骨骼的地方减少,但在远离骨骼的地方进一步扩展。 BM 去甲肾上腺素能神经支配的增加促进 β2-肾上腺素能受体 (AR)-白细胞介素 6 依赖性巨核细胞生成。 β3-AR-Nos1 活性降低与骨内膜微环境减少和巨核细胞与血窦并置有关。然而,用 β3-AR 激动剂长期治疗早衰小鼠会减少过早的骨髓和 HSC 扩张,并恢复 HSC 与巨核细胞的近端关联。因此,骨髓微环境的正常/过早衰老会促进骨髓扩张,并且可以通过针对微环境来改善。













































 京公网安备 11010802027423号
京公网安备 11010802027423号