当前位置:
X-MOL 学术
›
ChemBioChem
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Metabolites of the Anaerobic Degradation of n-Hexane by Denitrifying Betaproteobacterium Strain HxN1.
ChemBioChem ( IF 2.6 ) Pub Date : 2019-10-30 , DOI: 10.1002/cbic.201900375 Julian Küppers 1 , Nico Mitschke 2 , Simone Heyen 2 , Ralf Rabus 2 , Heinz Wilkes 2 , Jens Christoffers 1
ChemBioChem ( IF 2.6 ) Pub Date : 2019-10-30 , DOI: 10.1002/cbic.201900375 Julian Küppers 1 , Nico Mitschke 2 , Simone Heyen 2 , Ralf Rabus 2 , Heinz Wilkes 2 , Jens Christoffers 1
Affiliation
|
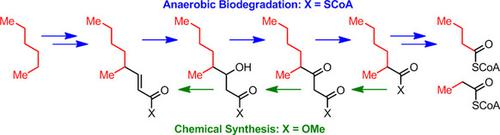
The constitutions of seven metabolites formed during anaerobic degradation of n-hexane by the denitrifying betaproteobacterium strain HxN1 were elucidated by comparison of their GC and MS data with those of synthetic reference standards. The synthesis of 4-methyloctanoic acid derivatives was accomplished by the conversion of 2-methylhexanoyl chloride with Meldrum's acid. The β-oxoester was reduced with NaBH4 , the hydroxy group was eliminated, and the double bond was displaced to yield the methyl esters of 4-methyl-3-oxooctanoate, 3-hydroxy-4-methyloctanoate, (E)-4-methyl-2-octenoate, and (E)- and (Z)-4-methyl-3-octenoate. The methyl esters of 2-methyl-3-oxohexanoate and 3-hydroxy-2-methylhexanoate were similarly prepared from butanoyl chloride and Meldrum's acid. However, methyl (E)-2-methyl-2-hexenoate was prepared by Horner-Wadsworth-Emmons reaction, followed by isomerization to methyl (E)-2-methyl-3-hexenoate. This investigation, with the exception of 4-methyl-3-oxooctanoate, which was not detectable in the cultures, completes the unambiguous identification of all intermediates of the anaerobic biodegradation of n-hexane to 2-methyl-3-oxohexanoyl coenzyme A (CoA), which is then thiolytically cleaved to butanoyl-CoA and propionyl-CoA; these two metabolites are further transformed according to established pathways.
中文翻译:

β-变形杆菌菌株HxN1反硝化,正己烷厌氧降解的代谢物。
通过反硝化β变形杆菌HxN1菌株在正己烷厌氧降解过程中形成的七种代谢物的组成,通过将它们的GC和MS数据与合成参考标准品进行了比较。4-甲基辛酸衍生物的合成通过将2-甲基己酰氯与麦德鲁姆酸转化而完成。用NaBH4还原β-氧代酸酯,消除羟基,并置换双键,生成4-甲基-3-氧代辛酸酯,3-羟基-4-甲基辛酸酯,(E)-4-甲基酯-2-辛烯酸酯,以及(E)-和(Z)-4-甲基-3-辛烯酸酯。类似地,由丁酰氯和Meldrum酸制备2-甲基-3-氧己酸酯和3-羟基-2-甲基己酸酯的甲酯。然而,通过Horner-Wadsworth-Emmons反应制备(E)-2-甲基-2-己烯酸甲酯,然后异构化为(E)-2-甲基-3-己烯酸甲酯。除了在培养物中无法检测到的4-甲基-3-氧代辛酸酯外,这项研究完成了对正己烷厌氧降解为2-甲基-3-氧代己酰基辅酶A(CoA)的所有中间体的明确鉴定。 ),然后将其硫解切割成丁酰基-CoA和丙酰基-CoA;这两种代谢物根据已建立的途径进一步转化。完成对正己烷厌氧生物降解为2-甲基-3-氧代己酸辅酶A(CoA)的所有中间体的明确鉴定,然后将其硫解裂解为丁酰基-CoA和丙酰基-CoA; 这两种代谢物根据已建立的途径进一步转化。完成对正己烷厌氧生物降解为2-甲基-3-氧代己酸辅酶A(CoA)的所有中间体的明确鉴定,然后将其硫解裂解为丁酰基-CoA和丙酰基-CoA; 这两种代谢物根据已建立的途径进一步转化。
更新日期:2019-10-30
中文翻译:

β-变形杆菌菌株HxN1反硝化,正己烷厌氧降解的代谢物。
通过反硝化β变形杆菌HxN1菌株在正己烷厌氧降解过程中形成的七种代谢物的组成,通过将它们的GC和MS数据与合成参考标准品进行了比较。4-甲基辛酸衍生物的合成通过将2-甲基己酰氯与麦德鲁姆酸转化而完成。用NaBH4还原β-氧代酸酯,消除羟基,并置换双键,生成4-甲基-3-氧代辛酸酯,3-羟基-4-甲基辛酸酯,(E)-4-甲基酯-2-辛烯酸酯,以及(E)-和(Z)-4-甲基-3-辛烯酸酯。类似地,由丁酰氯和Meldrum酸制备2-甲基-3-氧己酸酯和3-羟基-2-甲基己酸酯的甲酯。然而,通过Horner-Wadsworth-Emmons反应制备(E)-2-甲基-2-己烯酸甲酯,然后异构化为(E)-2-甲基-3-己烯酸甲酯。除了在培养物中无法检测到的4-甲基-3-氧代辛酸酯外,这项研究完成了对正己烷厌氧降解为2-甲基-3-氧代己酰基辅酶A(CoA)的所有中间体的明确鉴定。 ),然后将其硫解切割成丁酰基-CoA和丙酰基-CoA;这两种代谢物根据已建立的途径进一步转化。完成对正己烷厌氧生物降解为2-甲基-3-氧代己酸辅酶A(CoA)的所有中间体的明确鉴定,然后将其硫解裂解为丁酰基-CoA和丙酰基-CoA; 这两种代谢物根据已建立的途径进一步转化。完成对正己烷厌氧生物降解为2-甲基-3-氧代己酸辅酶A(CoA)的所有中间体的明确鉴定,然后将其硫解裂解为丁酰基-CoA和丙酰基-CoA; 这两种代谢物根据已建立的途径进一步转化。

















































 京公网安备 11010802027423号
京公网安备 11010802027423号