Nature Communications ( IF 14.7 ) Pub Date : 2019-07-11 , DOI: 10.1038/s41467-019-10940-4 Shenci Lu 1, 2 , Shawn Voon Hwee Ng 2 , Kaitlyn Lovato 3 , Jun-Yang Ong 2 , Si Bei Poh 2 , Xiao Qian Ng 2 , László Kürti 3 , Yu Zhao 2
|
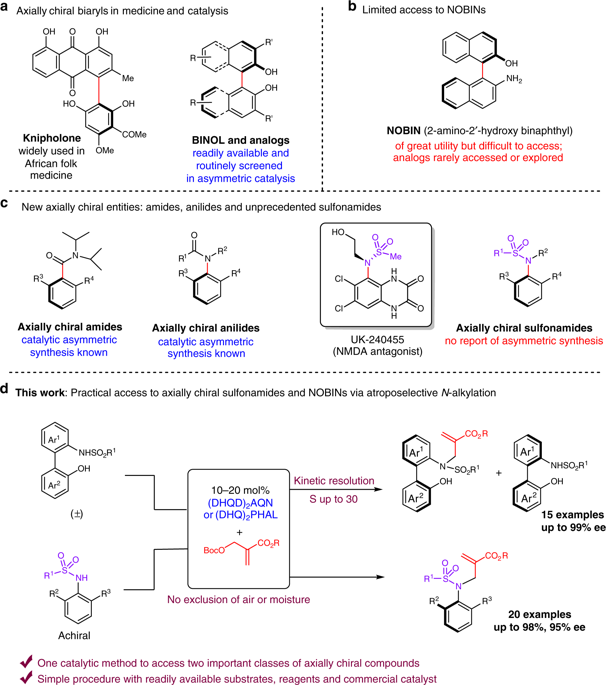
The importance of axial chirality in enantioselective synthesis has been widely recognized for decades. The practical access to certain structures such as biaryl amino phenols known as NOBINs in enantiopure form, however, still remains a challenge. In drug delivery, the incorporation of axially chiral molecules in systematic screening has also received a great deal of interest in recent years, which calls for innovation and practical synthesis of structurally different axially chiral entities. Herein we present an operationally simple catalytic N-alkylation of sulfonamides using commercially available chiral amine catalysts to deliver two important classes of axially chiral compounds: structurally diverse NOBIN analogs as well as axially chiral N-aryl sulfonamides in excellent enantiopurity. Structurally related chiral sulfonamide has shown great potential in drug molecules but enantioselective synthesis of them has never been accomplished before. The practical catalytic procedures of our methods also bode well for their wide application in enantioselective synthesis.
中文翻译:

通过有机催化的对位选择性N-烷基化可实际获得轴向手性磺酰胺和联芳基氨基苯酚。
数十年来,轴向手性在对映选择性合成中的重要性已得到广泛认可。然而,实际获得某些结构,例如对映纯形式的称为NOBIN的联芳基氨基苯酚仍然是一个挑战。在药物递送中,近年来,在系统筛选中掺入轴向手性分子也引起了极大的兴趣,这要求结构上不同的轴向手性实体的创新和实用合成。本文中,我们介绍了使用市售的手性胺催化剂对磺酰胺进行操作简单的催化N-烷基化反应,以提供两类重要的轴向手性化合物:结构上不同的NOBIN类似物以及轴向手性N优异的对映体纯度的-芳基磺酰胺类。与结构相关的手性磺酰胺在药物分子中显示出巨大潜力,但它们的对映选择性合成从未实现。我们的方法的实际催化程序也很适合在对映选择性合成中广泛应用。




















































 京公网安备 11010802027423号
京公网安备 11010802027423号