当前位置:
X-MOL 学术
›
Chemosphere
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Metal oxyanion removal from wastewater using manganese-oxidizing aerobic granular sludge.
Chemosphere ( IF 8.1 ) Pub Date : 2019-07-11 , DOI: 10.1016/j.chemosphere.2019.124353 Zhanfei He 1 , Zhen Wei 1 , Qingying Zhang 1 , Jinte Zou 1 , Xiangliang Pan 2
Chemosphere ( IF 8.1 ) Pub Date : 2019-07-11 , DOI: 10.1016/j.chemosphere.2019.124353 Zhanfei He 1 , Zhen Wei 1 , Qingying Zhang 1 , Jinte Zou 1 , Xiangliang Pan 2
Affiliation
|
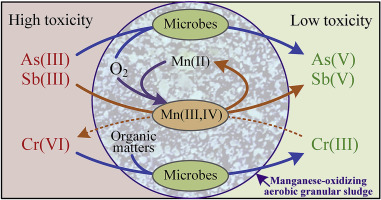
As, Sb, and Cr are redox-sensitive and toxic heavy metal(loid)s, and redox reactions are usually involved in the treatment of substrates containing these elements. In this study, manganese-oxidizing aerobic granular sludge (Mn-AGS) was obtained by continuously adding Mn(II) to the sludge in a sequencing batch reactor (SBR). Morphological observations, and analyses of extracellular polymeric substances (EPS), Mn valence-states, and microbial communities were performed on the resulting sludge. After 50 days of cultivation, biogenic Mn(III,IV) oxides (bio-MnOx) accumulated up to approximately 25 mg Mn/g suspended solids (SS). X-ray photoelectron spectroscopy (XPS) revealed that the percentage of Mn(III,IV) was 87.6%. The protein (PN) component in EPS increased from 80.3 to 87.8 mg/g volatile suspended solids (VSS) during cultivation, which might be favorable for sludge granulation and heavy metal(loid) removal. Batch experiments showed that Mn-AGS was better at oxidizing As(III)/Sb(III) into less toxic As(V)/Sb(V) than traditional AGS. Remarkably, the results indicated that Mn-AGS did not oxidize Cr(III) but was able to reduce Cr(VI) into relatively harmless Cr(III). This work provided a new promising method with which to treat As(III), Sb(III), and Cr(VI) in wastewaters.
中文翻译:

使用锰氧化的好氧颗粒污泥从废水中去除金属氧阴离子。
As,Sb和Cr是对氧化还原敏感且有毒的重金属(胶体),氧化还原反应通常涉及含有这些元素的底物的处理。在这项研究中,通过在定序分批反应器(SBR)中向污泥中连续添加Mn(II),获得了氧化锰的好氧颗粒污泥(Mn-AGS)。对产生的污泥进行形态学观察和细胞外聚合物(EPS),Mn价态和微生物群落的分析。培养50天后,生物型Mn(III,IV)氧化物(bio-MnOx)累积积累的最大量约为25 mg Mn / g悬浮固体(SS)。X射线光电子能谱(XPS)显示Mn(III,IV)的百分比为87.6%。在培养过程中,EPS中的蛋白质(PN)成分从80.3 mg / g增至87.8 mg / g挥发性悬浮固体(VSS),这可能有利于污泥的颗粒化和重金属的去除。批处理实验表明,与传统的AGS相比,Mn-AGS能够更好地将As(III)/ Sb(III)氧化为毒性较小的As(V)/ Sb(V)。值得注意的是,结果表明,Mn-AGS不会氧化Cr(III),但能够将Cr(VI)还原为相对无害的Cr(III)。这项工作提供了一种新的有前途的方法来处理废水中的As(III),Sb(III)和Cr(VI)。
更新日期:2019-07-11
中文翻译:

使用锰氧化的好氧颗粒污泥从废水中去除金属氧阴离子。
As,Sb和Cr是对氧化还原敏感且有毒的重金属(胶体),氧化还原反应通常涉及含有这些元素的底物的处理。在这项研究中,通过在定序分批反应器(SBR)中向污泥中连续添加Mn(II),获得了氧化锰的好氧颗粒污泥(Mn-AGS)。对产生的污泥进行形态学观察和细胞外聚合物(EPS),Mn价态和微生物群落的分析。培养50天后,生物型Mn(III,IV)氧化物(bio-MnOx)累积积累的最大量约为25 mg Mn / g悬浮固体(SS)。X射线光电子能谱(XPS)显示Mn(III,IV)的百分比为87.6%。在培养过程中,EPS中的蛋白质(PN)成分从80.3 mg / g增至87.8 mg / g挥发性悬浮固体(VSS),这可能有利于污泥的颗粒化和重金属的去除。批处理实验表明,与传统的AGS相比,Mn-AGS能够更好地将As(III)/ Sb(III)氧化为毒性较小的As(V)/ Sb(V)。值得注意的是,结果表明,Mn-AGS不会氧化Cr(III),但能够将Cr(VI)还原为相对无害的Cr(III)。这项工作提供了一种新的有前途的方法来处理废水中的As(III),Sb(III)和Cr(VI)。






























 京公网安备 11010802027423号
京公网安备 11010802027423号