当前位置:
X-MOL 学术
›
ChemistrySelect
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Water‐Mediated, Highly‐Efficient and Improved Protocol for the Synthesis of Vesamicol, Its Analogues and β‐Blockers through the Highly‐Chemoselective Aminolysis of Epoxides
ChemistrySelect ( IF 1.9 ) Pub Date : 2019-07-11 , DOI: 10.1002/slct.201901133 Jyoti Agarwal 1 , Rama Krishna Peddinti 2
ChemistrySelect ( IF 1.9 ) Pub Date : 2019-07-11 , DOI: 10.1002/slct.201901133 Jyoti Agarwal 1 , Rama Krishna Peddinti 2
Affiliation
|
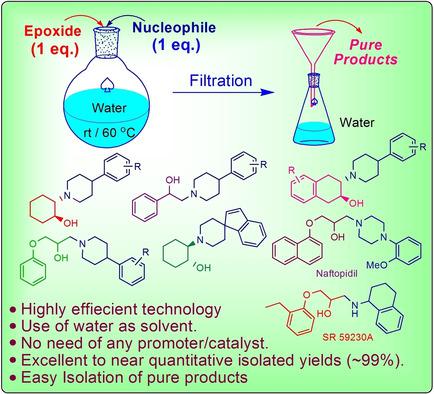
An efficient, eco‐friendly and cost‐effective protocol has been developed for the synthesis of vesamicol, benzovesamicol, spirovesamicol, their analogues and drug molecules such as Naftopidil and SR 59230 A. In this reaction, water has played dual role i. e. of i) bifunctional catalyst and ii) reaction medium. Except reactants, no other chemical reagent/promotor is required to afford the products. Reaction follows 100% atom economy and has been found to be highly chemo‐selective. All studied reactions worked well to yield the corresponding products in excellent to near quantitative yields without following the tedious and time consuming column chromatography as purification step. Thus, this protocol provides the vesamicol and other products without generating any chemical waste to the environment. Furthermore, this methodology has been successfully applied for 15 gm scale also.
中文翻译:

水介导,高效和改进的方案,用于通过环氧化物的高度化学选择性氨解来合成维他命,其类似物和β-阻滞剂
已开发出一种有效,环保且具有成本效益的方案,用于合成维他命醇,苯并维他命,螺螺维他命,其类似物和Naftopidil和SR 59230 A等药物分子。在该反应中,水起着双重作用。e。i)双功能催化剂和ii)反应介质。除反应物外,无需其他化学试剂/促进剂即可获得产品。反应遵循100%的原子经济性,已发现具有高度的化学选择性。所有研究的反应均能很好地完成,以优异的收率获得接近定量的相应产物,而无需将乏味且耗时的柱色谱法作为纯化步骤。因此,该协议可提供vesamicol和其他产品,而不会对环境产生任何化学废物。此外,该方法也已成功应用于15 gm规模。
更新日期:2019-07-11
中文翻译:

水介导,高效和改进的方案,用于通过环氧化物的高度化学选择性氨解来合成维他命,其类似物和β-阻滞剂
已开发出一种有效,环保且具有成本效益的方案,用于合成维他命醇,苯并维他命,螺螺维他命,其类似物和Naftopidil和SR 59230 A等药物分子。在该反应中,水起着双重作用。e。i)双功能催化剂和ii)反应介质。除反应物外,无需其他化学试剂/促进剂即可获得产品。反应遵循100%的原子经济性,已发现具有高度的化学选择性。所有研究的反应均能很好地完成,以优异的收率获得接近定量的相应产物,而无需将乏味且耗时的柱色谱法作为纯化步骤。因此,该协议可提供vesamicol和其他产品,而不会对环境产生任何化学废物。此外,该方法也已成功应用于15 gm规模。































 京公网安备 11010802027423号
京公网安备 11010802027423号