Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
SENP1-Sirt3 Signaling Controls Mitochondrial Protein Acetylation and Metabolism.
Molecular Cell ( IF 14.5 ) Pub Date : 2019-07-10 , DOI: 10.1016/j.molcel.2019.06.008 Tianshi Wang 1 , Ying Cao 1 , Quan Zheng 1 , Jun Tu 1 , Wei Zhou 1 , Jianli He 1 , Jie Zhong 2 , Yalan Chen 1 , Jiqiu Wang 3 , Rong Cai 1 , Yong Zuo 1 , Bo Wei 1 , Qiuju Fan 1 , Jie Yang 2 , Yicheng Wu 2 , Jing Yi 1 , Dali Li 4 , Mingyao Liu 4 , Chuangui Wang 5 , Aiwu Zhou 6 , Yu Li 7 , Xuefeng Wu 8 , Wen Yang 1 , Y Eugene Chin 9 , Guoqiang Chen 10 , Jinke Cheng 1
Molecular Cell ( IF 14.5 ) Pub Date : 2019-07-10 , DOI: 10.1016/j.molcel.2019.06.008 Tianshi Wang 1 , Ying Cao 1 , Quan Zheng 1 , Jun Tu 1 , Wei Zhou 1 , Jianli He 1 , Jie Zhong 2 , Yalan Chen 1 , Jiqiu Wang 3 , Rong Cai 1 , Yong Zuo 1 , Bo Wei 1 , Qiuju Fan 1 , Jie Yang 2 , Yicheng Wu 2 , Jing Yi 1 , Dali Li 4 , Mingyao Liu 4 , Chuangui Wang 5 , Aiwu Zhou 6 , Yu Li 7 , Xuefeng Wu 8 , Wen Yang 1 , Y Eugene Chin 9 , Guoqiang Chen 10 , Jinke Cheng 1
Affiliation
|
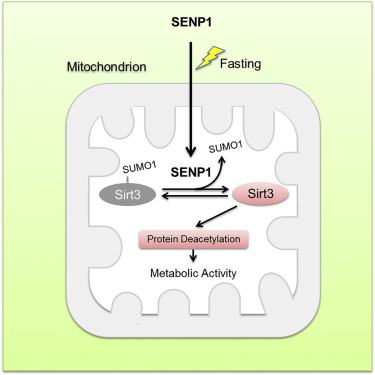
Sirt3, as a major mitochondrial nicotinamide adenine dinucleotide (NAD)-dependent deacetylase, is required for mitochondrial metabolic adaption to various stresses. However, how to regulate Sirt3 activity responding to metabolic stress remains largely unknown. Here, we report Sirt3 as a SUMOylated protein in mitochondria. SUMOylation suppresses Sirt3 catalytic activity. SUMOylation-deficient Sirt3 shows elevated deacetylation on mitochondrial proteins and increased fatty acid oxidation. During fasting, SUMO-specific protease SENP1 is accumulated in mitochondria and quickly de-SUMOylates and activates Sirt3. SENP1 deficiency results in hyper-SUMOylation of Sirt3 and hyper-acetylation of mitochondrial proteins, which reduces mitochondrial metabolic adaption responding to fasting. Furthermore, we find that fasting induces SENP1 translocation into mitochondria to activate Sirt3. The studies on mice show that Sirt3 SUMOylation mutation reduces fat mass and antagonizes high-fat diet (HFD)-induced obesity via increasing oxidative phosphorylation and energy expenditure. Our results reveal that SENP1-Sirt3 signaling modulates Sirt3 activation and mitochondrial metabolism during metabolic stress.
中文翻译:

SENP1-Sirt3信号传导控制线粒体蛋白质乙酰化和代谢。
作为主要的线粒体烟酰胺腺嘌呤二核苷酸(NAD)依赖性脱乙酰基酶,Sirt3是线粒体代谢适应各种压力所必需的。但是,如何调节Sirt3活性以应对代谢应激仍然是未知的。在这里,我们报告Sirt3作为线粒体中的SUMO化蛋白。SUMOylation抑制Sirt3催化活性。SUMOylation缺陷的Sirt3在线粒体蛋白上显示出更高的去乙酰化作用,并增加了脂肪酸的氧化作用。禁食期间,SUMO特异性蛋白酶SENP1积累在线粒体中,并迅速脱SUMOylate并激活Sirt3。SENP1缺乏会导致Sirt3的SUMO过度化和线粒体蛋白的超乙酰化,从而降低响应禁食的线粒体代谢适应性。此外,我们发现禁食会诱导SENP1易位到线粒体中,从而激活Sirt3。对小鼠的研究表明,Sirt3 SUMOylation突变可通过增加氧化磷酸化和增加能量消耗来减少脂肪量并拮抗高脂饮食(HFD)诱导的肥胖。我们的结果表明,SENP1-Sirt3信号传导在代谢应激过程中调节Sirt3激活和线粒体代谢。
更新日期:2019-07-10
中文翻译:

SENP1-Sirt3信号传导控制线粒体蛋白质乙酰化和代谢。
作为主要的线粒体烟酰胺腺嘌呤二核苷酸(NAD)依赖性脱乙酰基酶,Sirt3是线粒体代谢适应各种压力所必需的。但是,如何调节Sirt3活性以应对代谢应激仍然是未知的。在这里,我们报告Sirt3作为线粒体中的SUMO化蛋白。SUMOylation抑制Sirt3催化活性。SUMOylation缺陷的Sirt3在线粒体蛋白上显示出更高的去乙酰化作用,并增加了脂肪酸的氧化作用。禁食期间,SUMO特异性蛋白酶SENP1积累在线粒体中,并迅速脱SUMOylate并激活Sirt3。SENP1缺乏会导致Sirt3的SUMO过度化和线粒体蛋白的超乙酰化,从而降低响应禁食的线粒体代谢适应性。此外,我们发现禁食会诱导SENP1易位到线粒体中,从而激活Sirt3。对小鼠的研究表明,Sirt3 SUMOylation突变可通过增加氧化磷酸化和增加能量消耗来减少脂肪量并拮抗高脂饮食(HFD)诱导的肥胖。我们的结果表明,SENP1-Sirt3信号传导在代谢应激过程中调节Sirt3激活和线粒体代谢。































 京公网安备 11010802027423号
京公网安备 11010802027423号