当前位置:
X-MOL 学术
›
Nat. Struct. Mol. Biol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Structure of the eukaryotic protein O-mannosyltransferase Pmt1-Pmt2 complex.
Nature Structural & Molecular Biology ( IF 12.5 ) Pub Date : 2019-07-08 , DOI: 10.1038/s41594-019-0262-6 Lin Bai 1 , Amanda Kovach 1 , Qinglong You 1 , Alanna Kenny 1 , Huilin Li 1
Nature Structural & Molecular Biology ( IF 12.5 ) Pub Date : 2019-07-08 , DOI: 10.1038/s41594-019-0262-6 Lin Bai 1 , Amanda Kovach 1 , Qinglong You 1 , Alanna Kenny 1 , Huilin Li 1
Affiliation
|
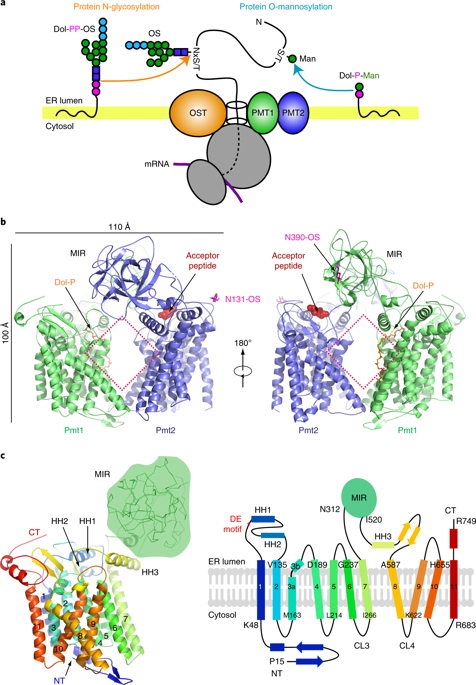
In eukaryotes, a nascent peptide entering the endoplasmic reticulum (ER) is scanned by two Sec61 translocon-associated large membrane machines for protein N-glycosylation and protein O-mannosylation, respectively. While the structure of the eight-protein oligosaccharyltransferase complex has been determined recently, the structures of mannosyltransferases of the PMT family, which are an integral part of ER protein homeostasis, are still unknown. Here we report cryo-EM structures of the Saccharomyces cerevisiae Pmt1-Pmt2 complex bound to a donor and an acceptor peptide at 3.2-Å resolution, showing that each subunit contains 11 transmembrane helices and a lumenal β-trefoil fold termed the MIR domain. The structures reveal the substrate recognition model and confirm an inverting mannosyl-transferring reaction mechanism by the enzyme complex. Furthermore, we found that the transmembrane domains of Pmt1 and Pmt2 share a structural fold with the catalytic subunits of oligosaccharyltransferases, confirming a previously proposed evolutionary relationship between protein O-mannosylation and protein N-glycosylation.
中文翻译:

真核蛋白O-甘露糖基转移酶Pmt1-Pmt2复合物的结构。
在真核生物中,进入内质网(ER)的新生肽被两个与Sec61 translocon相关的大型膜机扫描,分别用于蛋白N-糖基化和蛋白O-甘露糖基化。虽然最近已经确定了八蛋白寡糖基转移酶复合物的结构,但作为ER蛋白稳态的组成部分的PMT家族的甘露糖基转移酶的结构仍是未知的。在这里我们报告啤酒酵母Pmt1-Pmt2复杂绑定到供体和受体肽在3.2-Å分辨率的冷冻EM结构,表明每个亚基包含11个跨膜螺旋和称为MIR域的腔β-三叶折叠。这些结构揭示了底物识别模型,并通过酶复合物证实了逆转甘露糖基转移的反应机理。
更新日期:2019-07-09
中文翻译:

真核蛋白O-甘露糖基转移酶Pmt1-Pmt2复合物的结构。
在真核生物中,进入内质网(ER)的新生肽被两个与Sec61 translocon相关的大型膜机扫描,分别用于蛋白N-糖基化和蛋白O-甘露糖基化。虽然最近已经确定了八蛋白寡糖基转移酶复合物的结构,但作为ER蛋白稳态的组成部分的PMT家族的甘露糖基转移酶的结构仍是未知的。在这里我们报告啤酒酵母Pmt1-Pmt2复杂绑定到供体和受体肽在3.2-Å分辨率的冷冻EM结构,表明每个亚基包含11个跨膜螺旋和称为MIR域的腔β-三叶折叠。这些结构揭示了底物识别模型,并通过酶复合物证实了逆转甘露糖基转移的反应机理。































 京公网安备 11010802027423号
京公网安备 11010802027423号