当前位置:
X-MOL 学术
›
Anal. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Isolating Single Euglena gracilis Cells by Glass Microfluidics for Raman Analysis of Paramylon Biogenesis.
Analytical Chemistry ( IF 6.7 ) Pub Date : 2019-07-08 00:00:00 , DOI: 10.1021/acs.analchem.9b01007 Nobutoshi Ota 1 , Yusuke Yonamine 2 , Takuya Asai 3 , Yaxiaer Yalikun 1 , Takuro Ito 4, 5 , Yasuyuki Ozeki 3 , Yu Hoshino 6 , Yo Tanaka 1
Analytical Chemistry ( IF 6.7 ) Pub Date : 2019-07-08 00:00:00 , DOI: 10.1021/acs.analchem.9b01007 Nobutoshi Ota 1 , Yusuke Yonamine 2 , Takuya Asai 3 , Yaxiaer Yalikun 1 , Takuro Ito 4, 5 , Yasuyuki Ozeki 3 , Yu Hoshino 6 , Yo Tanaka 1
Affiliation
|
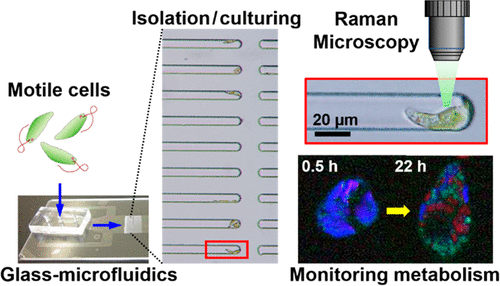
Time-course analysis of single cells is important to characterize heterogeneous activities of individual cells such as the metabolic response to their environment. Single-cell isolation is an essential step prior to time-course analysis of individual cells by collecting, culturing, and identifying multiple single-cell targets. Although single-cell isolation has been performed by various methods previously, a glass microfluidic device with semiclosed microchannels dramatically improved this process with its simple operation and easy transfer for time-course analysis of identified single cells. This study demonstrates isolating single cells of the highly motile microalgae, Euglena gracilis, by semiclosed microchannels with liquid flow only. The isolated single cells were identified in isolating channels and continuously cultured to track, by Raman microscopy, for the formation of subcellular granules composed of polysaccharide paramylon, a unique metabolite of E. gracilis, generated through photosynthesis. Through low-temperature glass bonding, a thin glass interface was incorporated to the microfluidic device. Thus, the device could perform the direct measurements of cultured single cells at high magnification by Raman microscopy with low background noise. In this study, the first demonstration of sequential monitoring of paramylon biogenesis in a single identified E. gracilis cell is shown.
中文翻译:

通过玻璃微流控技术分离单个眼虫细胞,用于拉曼分析副粘菌素的生物发生。
单个细胞的时程分析对于表征单个细胞的异质活性(例如对其环境的代谢反应)非常重要。通过收集,培养和识别多个单细胞靶标,单细胞分离是对单个细胞进行时程分析之前必不可少的步骤。尽管以前已经通过各种方法进行了单细胞分离,但是具有半封闭微通道的玻璃微流体设备以其简单的操作和易于转移的特性,极大地改善了这一过程,从而可以对鉴定出的单细胞进行时程分析。这项研究表明,分离出能动性极强的微藻Euglena gracilis的单个细胞,仅通过半封闭的微通道进行液体流动。在分离通道中鉴定出分离的单细胞,并通过拉曼显微镜进行连续培养以追踪由多糖对mylon组成的亚细胞颗粒的形成,多糖paramylon是通过光合作用产生的E. gracilis的独特代谢产物。通过低温玻璃粘合,将薄玻璃界面结合到微流体装置中。因此,该设备可以通过拉曼显微镜在高放大倍率下以低背景噪声对培养的单个细胞进行直接测量。在这项研究中,显示了在单个已鉴定的埃希氏锥虫细胞中顺序监测副粘菌素生物发生的第一个证明。
更新日期:2019-07-08
中文翻译:

通过玻璃微流控技术分离单个眼虫细胞,用于拉曼分析副粘菌素的生物发生。
单个细胞的时程分析对于表征单个细胞的异质活性(例如对其环境的代谢反应)非常重要。通过收集,培养和识别多个单细胞靶标,单细胞分离是对单个细胞进行时程分析之前必不可少的步骤。尽管以前已经通过各种方法进行了单细胞分离,但是具有半封闭微通道的玻璃微流体设备以其简单的操作和易于转移的特性,极大地改善了这一过程,从而可以对鉴定出的单细胞进行时程分析。这项研究表明,分离出能动性极强的微藻Euglena gracilis的单个细胞,仅通过半封闭的微通道进行液体流动。在分离通道中鉴定出分离的单细胞,并通过拉曼显微镜进行连续培养以追踪由多糖对mylon组成的亚细胞颗粒的形成,多糖paramylon是通过光合作用产生的E. gracilis的独特代谢产物。通过低温玻璃粘合,将薄玻璃界面结合到微流体装置中。因此,该设备可以通过拉曼显微镜在高放大倍率下以低背景噪声对培养的单个细胞进行直接测量。在这项研究中,显示了在单个已鉴定的埃希氏锥虫细胞中顺序监测副粘菌素生物发生的第一个证明。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号