当前位置:
X-MOL 学术
›
ACS Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
“Intelligent” Pt Catalysts Studied on High-Surface-Area CaTiO3 Films
ACS Catalysis ( IF 11.3 ) Pub Date : 2019-07-08 00:00:00 , DOI: 10.1021/acscatal.9b01278 Chao Lin 1 , Alexandre C. Foucher 2 , Yichen Ji 1 , Christopher D. Curran 3 , Eric A. Stach 2 , Steven McIntosh 3 , Raymond J. Gorte 1
ACS Catalysis ( IF 11.3 ) Pub Date : 2019-07-08 00:00:00 , DOI: 10.1021/acscatal.9b01278 Chao Lin 1 , Alexandre C. Foucher 2 , Yichen Ji 1 , Christopher D. Curran 3 , Eric A. Stach 2 , Steven McIntosh 3 , Raymond J. Gorte 1
Affiliation
|
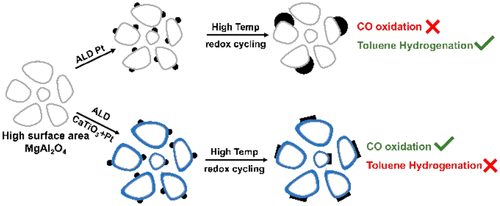
CaTiO3-supported Pt is sometimes referred to as an “Intelligent” catalyst because Pt can reversibly leave or enter the perovskite lattice following high-temperature reduction or oxidation; however, slow egress–ingress kinetics associated with large perovskite crystallites make these systems impractical. In the present work, thin films (∼1 nm) of CaTiO3 were deposited onto MgAl2O4 and then examined as catalyst supports for Pt and Pd. While Pd/CaTiO3/MgAl2O4 showed adsorption and CO-oxidation properties that were essentially the same as Pd/MgAl2O4, the Pt/CaTiO3/MgAl2O4 catalyst exhibited evidence for strong support interactions. Pt/CaTiO3/MgAl2O4 showed high activity for CO oxidation following reduction at 1073 K, even though CO adsorption was suppressed, but the catalysts were dramatically less active after oxidation at 1073 K and reduction at 773 K. Both Pt/CaTiO3/MgAl2O4 and a catalyst formed by ex-solution of CaTi0.95Pt0.05O3 exhibited very low rates for toluene hydrogenation in comparison to Pt/MgAl2O4. Scanning transmission electron microscopy (STEM) and energy-dispersive spectroscopy (EDS) showed that the CaTiO3 films uniformly covered the MgAl2O4 surface after both reduction and oxidation at 1073 K. Pt particles on reduced Pt/CaTiO3/MgAl2O4 exhibited an unusual rhombohedral shape and may be flat, a further indication of strong interactions between the metal and the support. Low-energy ion scattering (LEIS) indicated that high-temperature reduction caused a restructuring of the CaTiO3. The implications of these results for understanding catalysts formed by ex-solution of metals from a perovskite lattice are discussed.
中文翻译:

“智能”铂金催化剂研究了高表面积的CaTiO 3部电影
CaTiO 3负载的Pt有时被称为“智能”催化剂,因为在高温还原或氧化后Pt可以可逆地离开或进入钙钛矿晶格。但是,与大型钙钛矿微晶相关的缓慢的进-进动力学使这些系统不切实际。在目前的工作中,将CaTiO 3薄膜(约1 nm)沉积在MgAl 2 O 4上,然后作为Pt和Pd的催化剂载体进行检查。尽管Pd / CaTiO 3 / MgAl 2 O 4的吸附和CO氧化性质与Pd / MgAl 2 O 4,Pt / CaTiO 3 / MgAl 2基本上相同O 4催化剂显示出强的载体相互作用的证据。Pt / CaTiO 3 / MgAl 2 O 4在1073 K下还原后对CO氧化表现出高活性,即使CO吸附被抑制,但在1073 K下氧化并在773 K下还原后,催化剂的活性也大大降低。与Pt / MgAl 2 O 4相比,由CaTi 0.95 Pt 0.05 O 3的原溶液形成的催化剂3 / MgAl 2 O 4和甲苯的氢化率非常低。。扫描透射电子显微镜(STEM)和能量分散光谱(EDS)表明,CaTiO 3薄膜在1073 K下还原和氧化后均均匀地覆盖了MgAl 2 O 4表面。还原后的Pt / CaTiO 3 / MgAl 2 O上的Pt颗粒图4显示出不寻常的菱形形状并且可以是平坦的,这进一步表明金属与载体之间强烈的相互作用。低能离子散射(LEIS)表明高温还原引起CaTiO 3的重组。讨论了这些结果对于理解由钙钛矿晶格中的金属进行固溶而形成的催化剂的意义。
更新日期:2019-07-08
中文翻译:

“智能”铂金催化剂研究了高表面积的CaTiO 3部电影
CaTiO 3负载的Pt有时被称为“智能”催化剂,因为在高温还原或氧化后Pt可以可逆地离开或进入钙钛矿晶格。但是,与大型钙钛矿微晶相关的缓慢的进-进动力学使这些系统不切实际。在目前的工作中,将CaTiO 3薄膜(约1 nm)沉积在MgAl 2 O 4上,然后作为Pt和Pd的催化剂载体进行检查。尽管Pd / CaTiO 3 / MgAl 2 O 4的吸附和CO氧化性质与Pd / MgAl 2 O 4,Pt / CaTiO 3 / MgAl 2基本上相同O 4催化剂显示出强的载体相互作用的证据。Pt / CaTiO 3 / MgAl 2 O 4在1073 K下还原后对CO氧化表现出高活性,即使CO吸附被抑制,但在1073 K下氧化并在773 K下还原后,催化剂的活性也大大降低。与Pt / MgAl 2 O 4相比,由CaTi 0.95 Pt 0.05 O 3的原溶液形成的催化剂3 / MgAl 2 O 4和甲苯的氢化率非常低。。扫描透射电子显微镜(STEM)和能量分散光谱(EDS)表明,CaTiO 3薄膜在1073 K下还原和氧化后均均匀地覆盖了MgAl 2 O 4表面。还原后的Pt / CaTiO 3 / MgAl 2 O上的Pt颗粒图4显示出不寻常的菱形形状并且可以是平坦的,这进一步表明金属与载体之间强烈的相互作用。低能离子散射(LEIS)表明高温还原引起CaTiO 3的重组。讨论了这些结果对于理解由钙钛矿晶格中的金属进行固溶而形成的催化剂的意义。































 京公网安备 11010802027423号
京公网安备 11010802027423号