当前位置:
X-MOL 学术
›
ACS Med. Chem. Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Rotational Freedom, Steric Hindrance, and Protein Dynamics Explain BLU554 Selectivity for the Hinge Cysteine of FGFR4.
ACS Medicinal Chemistry Letters ( IF 3.5 ) Pub Date : 2019-07-03 00:00:00 , DOI: 10.1021/acsmedchemlett.9b00196
Xiaojing Lin 1 , Yuliana Yosaatmadja 1 , Maria Kalyukina 1 , Martin J Middleditch 1 , Zhen Zhang 2 , Xiaoyun Lu 2 , Ke Ding 2 , Adam V Patterson 3, 4 , Jeff B Smaill 3, 4 , Christopher J Squire 1, 4
ACS Medicinal Chemistry Letters ( IF 3.5 ) Pub Date : 2019-07-03 00:00:00 , DOI: 10.1021/acsmedchemlett.9b00196
Xiaojing Lin 1 , Yuliana Yosaatmadja 1 , Maria Kalyukina 1 , Martin J Middleditch 1 , Zhen Zhang 2 , Xiaoyun Lu 2 , Ke Ding 2 , Adam V Patterson 3, 4 , Jeff B Smaill 3, 4 , Christopher J Squire 1, 4
Affiliation
|
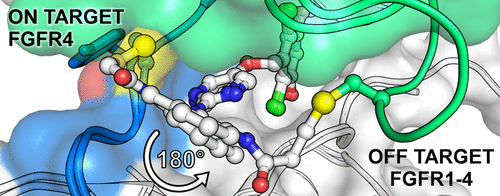
Aberration in FGFR4 signaling drives carcinogenesis and progression in a subset of hepatocellular carcinoma (HCC) patients, thereby making FGFR4 an attractive molecular target for this disease. Selective FGFR4 inhibition can be achieved through covalently targeting a poorly conserved cysteine residue in the FGFR4 kinase domain. We report mass spectrometry assays and cocrystal structures of FGFR4 in covalent complex with the clinical candidate BLU554 and with a series of four structurally related inhibitors that define the inherent reactivity and selectivity profile of these molecules. We further reveal the structure of FGFR1 with one of our inhibitors and show that off-target covalent binding can occur through an alternative conformation that supports targeting of a cysteine conserved in all members of the FGFR family. Collectively, we propose that rotational freedom, steric hindrance, and protein dynamics explain the exceptional selectivity profile of BLU554 for targeting FGFR4.
中文翻译:

旋转自由度,立体位阻和蛋白质动力学解释了BLU554对FGFR4铰链半胱氨酸的选择性。
FGFR4信号转导异常会驱动一部分肝细胞癌(HCC)患者的癌变和进展,从而使FGFR4成为该疾病的诱人分子靶标。可以通过共价靶向FGFR4激酶结构域中保守性较差的半胱氨酸残基来实现选择性FGFR4抑制。我们报告质谱分析和共价复合物与临床候选BLU554和一系列定义这些分子的固有反应性和选择性的四个结构相关的抑制剂共价复合物中的FGFR4的共晶体结构。我们进一步揭示了一种与我们的抑制剂结合的FGFR1的结构,并表明脱靶共价结合可以通过另一种构象发生,该构象支持FGFR家族所有成员中保守的半胱氨酸的靶向。总的来说,
更新日期:2019-07-03
中文翻译:

旋转自由度,立体位阻和蛋白质动力学解释了BLU554对FGFR4铰链半胱氨酸的选择性。
FGFR4信号转导异常会驱动一部分肝细胞癌(HCC)患者的癌变和进展,从而使FGFR4成为该疾病的诱人分子靶标。可以通过共价靶向FGFR4激酶结构域中保守性较差的半胱氨酸残基来实现选择性FGFR4抑制。我们报告质谱分析和共价复合物与临床候选BLU554和一系列定义这些分子的固有反应性和选择性的四个结构相关的抑制剂共价复合物中的FGFR4的共晶体结构。我们进一步揭示了一种与我们的抑制剂结合的FGFR1的结构,并表明脱靶共价结合可以通过另一种构象发生,该构象支持FGFR家族所有成员中保守的半胱氨酸的靶向。总的来说,

































 京公网安备 11010802027423号
京公网安备 11010802027423号