当前位置:
X-MOL 学术
›
J. Med. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Discovery of 3-Oxabicyclo[4.1.0]heptane, a Non-nitrogen Containing Morpholine Isostere, and Its Application in Novel Inhibitors of the PI3K-AKT-mTOR Pathway.
Journal of Medicinal Chemistry ( IF 6.8 ) Pub Date : 2019-07-23 , DOI: 10.1021/acs.jmedchem.9b00348 Heather Hobbs 1 , Gianpaolo Bravi 1 , Ian Campbell 1 , Maire Convery 1 , Hannah Davies 1 , Graham Inglis 1 , Sandeep Pal 1 , Simon Peace 1 , Joanna Redmond 1 , Declan Summers 1
Journal of Medicinal Chemistry ( IF 6.8 ) Pub Date : 2019-07-23 , DOI: 10.1021/acs.jmedchem.9b00348 Heather Hobbs 1 , Gianpaolo Bravi 1 , Ian Campbell 1 , Maire Convery 1 , Hannah Davies 1 , Graham Inglis 1 , Sandeep Pal 1 , Simon Peace 1 , Joanna Redmond 1 , Declan Summers 1
Affiliation
|
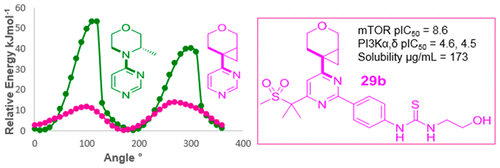
4-(Pyrimidin-4-yl)morpholines are privileged pharmacophores for PI3K and PIKKs inhibition by virtue of the morpholine oxygen, both forming the key hydrogen bonding interaction and conveying selectivity over the broader kinome. Key to the morpholine utility as a kinase hinge binder is its ability to adopt a coplanar conformation with an adjacent aromatic core favored by the morpholine nitrogen nonbonding pair of electrons interacting with the electron deficient pyrimidine π-system. Few selective morpholine replacements have been identified to date. Herein we describe the discovery of a potent non-nitrogen containing morpholine isostere with the ability to mimic this conformation and its application in a potent selective dual inhibitor of mTORC1 and mTORC2 (29b).
中文翻译:

3-氮杂双环[4.1.0]庚烷的发现,它在PI3K-AKT-mTOR途径的新型抑制剂中的应用。
4-(嘧啶-4-基)吗啉由于吗啉氧而成为抑制PI3K和PIKKs的特权药效基团,既形成了关键的氢键相互作用,又在更广泛的kinome上传递了选择性。吗啉作为激酶铰链结合剂的关键在于它能够与相邻的芳香核形成共面构象,而该芳香核受吗啉氮非键电子与缺电子的嘧啶π系统相互作用而受到促进。迄今为止,几乎没有发现选择性吗啉替代品。本文中,我们描述了一种具有强大的非氮的吗啉类异构体的能力,该异构体具有模仿这种构象的能力,并将其应用在mTORC1和mTORC2的有效选择性双重抑制剂中(29b)。
更新日期:2019-07-08
中文翻译:

3-氮杂双环[4.1.0]庚烷的发现,它在PI3K-AKT-mTOR途径的新型抑制剂中的应用。
4-(嘧啶-4-基)吗啉由于吗啉氧而成为抑制PI3K和PIKKs的特权药效基团,既形成了关键的氢键相互作用,又在更广泛的kinome上传递了选择性。吗啉作为激酶铰链结合剂的关键在于它能够与相邻的芳香核形成共面构象,而该芳香核受吗啉氮非键电子与缺电子的嘧啶π系统相互作用而受到促进。迄今为止,几乎没有发现选择性吗啉替代品。本文中,我们描述了一种具有强大的非氮的吗啉类异构体的能力,该异构体具有模仿这种构象的能力,并将其应用在mTORC1和mTORC2的有效选择性双重抑制剂中(29b)。







































 京公网安备 11010802027423号
京公网安备 11010802027423号