当前位置:
X-MOL 学术
›
Chem. Eur. J.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Synthesis of 1,4-Thiazepane-Based Curcuminoids with Promising Anticancer Activity.
Chemistry - A European Journal ( IF 3.9 ) Pub Date : 2019-09-03 , DOI: 10.1002/chem.201902549 Atiruj Theppawong 1 , Tim Van de Walle 1 , Kristof Van Hecke 2 , Charlotte Grootaert 3 , John Van Camp 3 , Matthias D'hooghe 1
Chemistry - A European Journal ( IF 3.9 ) Pub Date : 2019-09-03 , DOI: 10.1002/chem.201902549 Atiruj Theppawong 1 , Tim Van de Walle 1 , Kristof Van Hecke 2 , Charlotte Grootaert 3 , John Van Camp 3 , Matthias D'hooghe 1
Affiliation
|
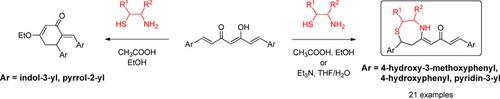
Curcumin, the main component of turmeric (Curcuma longa) is known to display an interesting bioactivity profile, including pronounced anticancer properties. However, its low bioavailability, metabolic instability and nonspecific activity are concerns that have to be addressed before curcuminoids can be considered for therapeutic applications. Within that framework, intensive research has been carried out in the last decades to develop new curcumin derivatives, generally centered on standard modifications of the sp2 curcumin framework, with the aim to augment its bioavailability while maintaining or improving its anticancer properties. To find potential hit molecules by moving away from the classical flat curcumin framework, we investigated an unexplored modification to produce novel, out-of-plane 1,4-thiazepane-based curcuminoids and assessed the impact of this modification on the biological activity. In this way, 21 new, structurally diverse thiazepane scaffolds (4-aryl-1-(7-aryl-1,4-thiazepan-5-ylidene)but-3-en-2-ones) were synthesized, as well as some biologically interesting unexpected reaction products (such as 5-aryl-6-arylmethylene-3-ethoxycyclohex-2-en-1-ones and 4-acetyl-5-aryl-2-(3-arylacryloyl)-3-methylcyclohex-2-en-1-ones). All these analogues were subsequently tested on their antioxidant capacity, their cytotoxicity properties and their ROS (reactive oxygen species) production. Many compounds demonstrated interesting activities, with ten curcuminoids, whereof eight 1,4-thiazepane-based, showing better antiproliferative properties compared to their mother compounds, as well as an increased ROS production. This unprecedented 3D curcumin modification has thus delivered promising new hit compounds with good activity profiles eligible for further exploration.
中文翻译:

合成具有前途的抗癌活性的1,4噻吩基姜黄素。
姜黄素(姜黄(姜黄)的主要成分)具有令人感兴趣的生物活性,包括明显的抗癌特性。然而,在将姜黄素考虑用于治疗应用之前,必须解决其低生物利用度,代谢不稳定和非特异性活性的问题。在该框架内,近几十年来进行了深入研究以开发新的姜黄素衍生物,通常以sp2姜黄素框架的标准修饰为中心,目的是在保持或改善其抗癌特性的同时提高其生物利用度。为了通过远离经典的扁平姜黄素框架来发现潜在的命中分子,我们研究了一种未经探索的修饰,以产生新颖的平面外1,基于4-噻嗪类的姜黄素,并评估了这种修饰对生物活性的影响。以这种方式,合成了21种结构上不同的新的硫氮杂支架(4-芳基-1-(7-芳基-1,4-噻氮杂-5-亚烷基)丁-3-烯-2-酮),以及一些具有生物学意义的意外反应产物(例如5-芳基-6-芳基亚甲基-3-乙氧基环己基-2-烯-1-酮和4-乙酰基-5-芳基-2-(3-芳基丙烯酰基)-3-甲基环己基-2- en-1个)。随后测试了所有这些类似物的抗氧化能力,细胞毒性以及ROS(活性氧)的产生。许多化合物表现出令人感兴趣的活性,其中有十种姜黄素类化合物,其中八种以1,4-硫氮烷为基础,与它们的母体化合物相比,显示出更好的抗增殖性能,并且活性氧的产生增加。
更新日期:2019-09-03
中文翻译:

合成具有前途的抗癌活性的1,4噻吩基姜黄素。
姜黄素(姜黄(姜黄)的主要成分)具有令人感兴趣的生物活性,包括明显的抗癌特性。然而,在将姜黄素考虑用于治疗应用之前,必须解决其低生物利用度,代谢不稳定和非特异性活性的问题。在该框架内,近几十年来进行了深入研究以开发新的姜黄素衍生物,通常以sp2姜黄素框架的标准修饰为中心,目的是在保持或改善其抗癌特性的同时提高其生物利用度。为了通过远离经典的扁平姜黄素框架来发现潜在的命中分子,我们研究了一种未经探索的修饰,以产生新颖的平面外1,基于4-噻嗪类的姜黄素,并评估了这种修饰对生物活性的影响。以这种方式,合成了21种结构上不同的新的硫氮杂支架(4-芳基-1-(7-芳基-1,4-噻氮杂-5-亚烷基)丁-3-烯-2-酮),以及一些具有生物学意义的意外反应产物(例如5-芳基-6-芳基亚甲基-3-乙氧基环己基-2-烯-1-酮和4-乙酰基-5-芳基-2-(3-芳基丙烯酰基)-3-甲基环己基-2- en-1个)。随后测试了所有这些类似物的抗氧化能力,细胞毒性以及ROS(活性氧)的产生。许多化合物表现出令人感兴趣的活性,其中有十种姜黄素类化合物,其中八种以1,4-硫氮烷为基础,与它们的母体化合物相比,显示出更好的抗增殖性能,并且活性氧的产生增加。












































 京公网安备 11010802027423号
京公网安备 11010802027423号