当前位置:
X-MOL 学术
›
J. Chem. Eng. Data
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Determination and Correlation for the Solubilities of Succinic Acid in Cyclohexanol + Cyclohexanone + Cyclohexane Solvent Mixtures
Journal of Chemical & Engineering Data ( IF 2.0 ) Pub Date : 2018-02-22 00:00:00 , DOI: 10.1021/acs.jced.7b00956 Xiaoxiao Sheng 1 , Weiping Luo 1 , Qinbo Wang 2
Journal of Chemical & Engineering Data ( IF 2.0 ) Pub Date : 2018-02-22 00:00:00 , DOI: 10.1021/acs.jced.7b00956 Xiaoxiao Sheng 1 , Weiping Luo 1 , Qinbo Wang 2
Affiliation
|
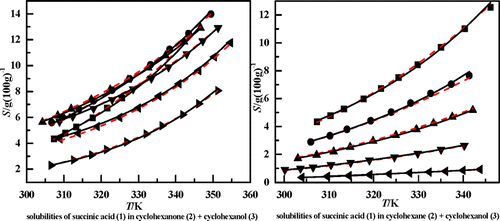
The solubilities of succinic acid in binary cyclohexanone + cyclohexanol solvent mixtures at 304.25–354.65 K, in binary cyclohexane + cyclohexanol solvent mixtures at 300.15–346.15 K, in binary cyclohexane + cyclohexanone solvent mixtures at 297.65–351.25 K, and in ternary cyclohexanol + cyclohexanone + cyclohexane solvent mixtures at 306.95–343.15 K were determined by the synthetic method at atmospheric pressure. The experimental solubility data in binary solvent mixtures were correlated by the Apelblat equation and nonrandom two-liquid (NRTL) activity coefficient model, and the calculated solubility data by the models have a good agreement with the experimental data. Then, the solubilities of succinic acid in ternary cyclohexanol + cyclohexanone + cyclohexane solvent mixtures were predicted by the NRTL model and compared with the experimental solubility data. Finally, the solubilities of succinic acid in the three studied binary solvent mixtures were compared with the solubilities of glutaric acid and adipic acid in the same solvent systems at 303.15–343.15 K. The result shows that in the three studied solvent mixtures, glutaric acid with an odd number of carbon atoms is much more soluble than succinic acid and adipic acid with an even number of carbon atoms. The reasons for this “odd–even effect” phenomenon are the interlayer packing of molecule chains and the twist of the carbon chains.
中文翻译:

琥珀酸在环己醇+环己酮+环己烷溶剂混合物中的溶解度测定及相关性
琥珀酸在304.25–354.65 K的二元环己酮+环己醇溶剂混合物中,在300.15–346.15 K的二元环己烷+环己醇溶剂混合物中,在297.65–351.25 K的二元环己烷+环己酮溶剂混合物中以及在三元环己醇+环己酮中的溶解度通过大气压下的合成方法测定了306.95–343.15 K下的+环己烷溶剂混合物。通过Apelblat方程和非随机二液(NRTL)活度系数模型对二元溶剂混合物中的实验溶解度数据进行了关联,该模型计算出的溶解度数据与实验数据具有很好的一致性。然后,NRTL模型预测了琥珀酸在环己醇+环己酮+环己烷三元混合物中的溶解度,并与实验溶解度数据进行了比较。最后,将丁二酸在三种研究的二元溶剂混合物中的溶解度与戊二酸和己二酸在相同溶剂体系中于303.15–343.15 K的溶解度进行了比较。结果表明,在这三种研究的混合物中,戊二酸与奇数个碳原子比具有偶数个碳原子的琥珀酸和己二酸可溶得多。这种“奇偶效应”现象的原因是分子链的层间堆积和碳链的扭曲。比较了在三种研究的二元溶剂混合物中琥珀酸的溶解度与在相同溶剂体系中303.15–343.15 K时戊二酸和己二酸的溶解度。结果表明,在这三种研究的溶剂混合物中,戊二酸与奇数碳原子数比具有偶数碳原子的琥珀酸和己二酸更易溶。这种“奇偶效应”现象的原因是分子链的层间堆积和碳链的扭曲。比较了在三种研究的二元溶剂混合物中琥珀酸的溶解度与在相同溶剂体系中303.15–343.15 K时戊二酸和己二酸的溶解度。结果表明,在这三种研究的溶剂混合物中,戊二酸与奇数碳原子数比具有偶数碳原子的琥珀酸和己二酸更易溶。这种“奇偶效应”现象的原因是分子链的层间堆积和碳链的扭曲。
更新日期:2018-02-23
中文翻译:

琥珀酸在环己醇+环己酮+环己烷溶剂混合物中的溶解度测定及相关性
琥珀酸在304.25–354.65 K的二元环己酮+环己醇溶剂混合物中,在300.15–346.15 K的二元环己烷+环己醇溶剂混合物中,在297.65–351.25 K的二元环己烷+环己酮溶剂混合物中以及在三元环己醇+环己酮中的溶解度通过大气压下的合成方法测定了306.95–343.15 K下的+环己烷溶剂混合物。通过Apelblat方程和非随机二液(NRTL)活度系数模型对二元溶剂混合物中的实验溶解度数据进行了关联,该模型计算出的溶解度数据与实验数据具有很好的一致性。然后,NRTL模型预测了琥珀酸在环己醇+环己酮+环己烷三元混合物中的溶解度,并与实验溶解度数据进行了比较。最后,将丁二酸在三种研究的二元溶剂混合物中的溶解度与戊二酸和己二酸在相同溶剂体系中于303.15–343.15 K的溶解度进行了比较。结果表明,在这三种研究的混合物中,戊二酸与奇数个碳原子比具有偶数个碳原子的琥珀酸和己二酸可溶得多。这种“奇偶效应”现象的原因是分子链的层间堆积和碳链的扭曲。比较了在三种研究的二元溶剂混合物中琥珀酸的溶解度与在相同溶剂体系中303.15–343.15 K时戊二酸和己二酸的溶解度。结果表明,在这三种研究的溶剂混合物中,戊二酸与奇数碳原子数比具有偶数碳原子的琥珀酸和己二酸更易溶。这种“奇偶效应”现象的原因是分子链的层间堆积和碳链的扭曲。比较了在三种研究的二元溶剂混合物中琥珀酸的溶解度与在相同溶剂体系中303.15–343.15 K时戊二酸和己二酸的溶解度。结果表明,在这三种研究的溶剂混合物中,戊二酸与奇数碳原子数比具有偶数碳原子的琥珀酸和己二酸更易溶。这种“奇偶效应”现象的原因是分子链的层间堆积和碳链的扭曲。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号