Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Computational and Experimental Druggability Assessment of Human DNA Glycosylases
ACS Omega ( IF 3.7 ) Pub Date : 2019-07-05 00:00:00 , DOI: 10.1021/acsomega.9b00162 Maurice Michel 1 , Torkild Visnes 1, 2 , Evert J. Homan 1 , Brinton Seashore-Ludlow 3 , Mattias Hedenström 4 , Elisée Wiita 1 , Karl Vallin 1 , Cynthia B. J. Paulin 1 , Jiaxi Zhang 5 , Olov Wallner 1 , Martin Scobie 1 , Andreas Schmidt 5 , Annika Jenmalm-Jensen 3 , Ulrika Warpman Berglund 1 , Thomas Helleday 1, 6
ACS Omega ( IF 3.7 ) Pub Date : 2019-07-05 00:00:00 , DOI: 10.1021/acsomega.9b00162 Maurice Michel 1 , Torkild Visnes 1, 2 , Evert J. Homan 1 , Brinton Seashore-Ludlow 3 , Mattias Hedenström 4 , Elisée Wiita 1 , Karl Vallin 1 , Cynthia B. J. Paulin 1 , Jiaxi Zhang 5 , Olov Wallner 1 , Martin Scobie 1 , Andreas Schmidt 5 , Annika Jenmalm-Jensen 3 , Ulrika Warpman Berglund 1 , Thomas Helleday 1, 6
Affiliation
|
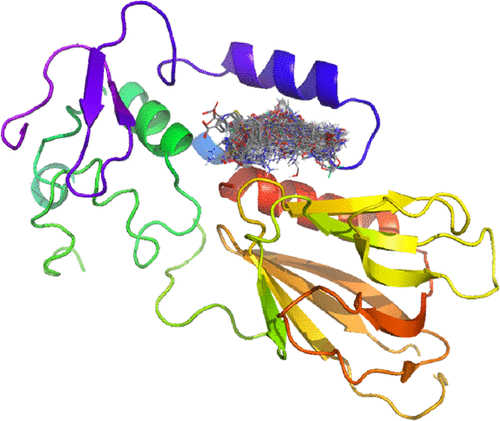
Due to a polar or even charged binding interface, DNA-binding proteins are considered extraordinarily difficult targets for development of small-molecule ligands and only a handful of proteins have been targeted successfully to date. Recently, however, it has been shown that development of selective and efficient inhibitors of 8-oxoguanine DNA glycosylase is possible. Here, we describe the initial druggability assessment of DNA glycosylases in a computational setting and experimentally investigate several methods to target endonuclease VIII-like 1 (NEIL1) with small-molecule inhibitors. We find that DNA glycosylases exhibit good predicted druggability in both DNA-bound and -unbound states. Furthermore, we find catalytic sites to be highly flexible, allowing for a range of interactions and binding partners. One flexible catalytic site was rationalized for NEIL1 and further investigated experimentally using both a biochemical assay in the presence of DNA and a thermal shift assay in the absence of DNA.
中文翻译:

人类DNA糖基化酶的计算和实验可药用性评估
由于极性或什至带电的结合界面,DNA结合蛋白被认为是开发小分子配体的极其困难的靶标,迄今为止只有极少数的蛋白被成功靶向。但是,最近显示出有可能开发选择性和有效的8-氧代鸟嘌呤DNA糖基化酶抑制剂。在这里,我们描述了在计算环境中对DNA糖基化酶的初始可药物性评估,并通过实验研究了使用小分子抑制剂靶向核酸内切酶VIII-like 1(NEIL1)的几种方法。我们发现,DNA糖基化酶在DNA结合和非结合状态均显示出良好的预测药物作用。此外,我们发现催化位点具有高度的灵活性,可以实现多种相互作用和结合伴侣。
更新日期:2019-07-05
中文翻译:

人类DNA糖基化酶的计算和实验可药用性评估
由于极性或什至带电的结合界面,DNA结合蛋白被认为是开发小分子配体的极其困难的靶标,迄今为止只有极少数的蛋白被成功靶向。但是,最近显示出有可能开发选择性和有效的8-氧代鸟嘌呤DNA糖基化酶抑制剂。在这里,我们描述了在计算环境中对DNA糖基化酶的初始可药物性评估,并通过实验研究了使用小分子抑制剂靶向核酸内切酶VIII-like 1(NEIL1)的几种方法。我们发现,DNA糖基化酶在DNA结合和非结合状态均显示出良好的预测药物作用。此外,我们发现催化位点具有高度的灵活性,可以实现多种相互作用和结合伴侣。































 京公网安备 11010802027423号
京公网安备 11010802027423号