Bioorganic & Medicinal Chemistry Letters ( IF 2.5 ) Pub Date : 2019-07-04 , DOI: 10.1016/j.bmcl.2019.06.062 Hiroyuki Miyachi 1 , Tomohiro Yuzuriha 2 , Ryotaro Tabata 2 , Syohei Fukuda 2 , Kazuto Nunomura 2 , Bangzhong Lin 2 , Tadayuki Kobayashi 2 , Kenji Ishimoto 2 , Takefumi Doi 2 , Keisuke Tachibana 2
|
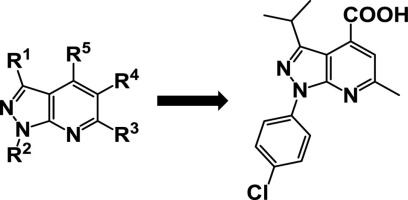
We previously reported that 1H-pyrazolo-[3,4-b]pyridine-4-carboxylic acid derivative 6 is an agonist of human peroxisome proliferator-activated receptor alpha (hPPARα). Here, we prepared a series of 1H-pyrazolo-[3,4-b]pyridine-4-carboxylic acid derivatives in order to examine the structure-activity relationships (SAR). SAR studies clearly indicated that the steric bulkiness of the substituent on 1H-pyrazolo-[3,4-b]pyridine ring, the position of the distal hydrophobic tail part, and the distance between the distal hydrophobic tail part and the acidic head part are critical for hPPARα agonistic activity. These SAR results are somewhat different from those reported for fibrate-class hPPARα agonists. A representative compound (10f) was as effective as fenofibrate in reducing the elevated plasma triglyceride levels in a high-fructose-fed rat model.
中文翻译:

1H-吡唑并-[3,4-b]吡啶-4-羧酸衍生物作为人过氧化物酶体增殖物激活受体α(PPARα)选择性激动剂的结构开发。
我们以前报道过1 H-吡唑并-[3,4- b ]吡啶-4-羧酸衍生物6是人过氧化物酶体增殖物激活受体α(hPPARα)的激动剂。在这里,我们准备了一系列1 H-吡唑并-[3,4- b ]吡啶-4-羧酸衍生物,以检查结构-活性关系(SAR)。SAR研究清楚表明,1 H-吡唑并-[3,4- b吡啶环,疏水性末端尾部的位置以及疏水性末端尾部与酸性头部之间的距离对于hPPARα激动活性至关重要。这些SAR结果与贝特类hPPARα激动剂报道的结果有些不同。在降低由高果糖喂养的大鼠模型中升高的血浆甘油三酸酯水平方面,代表性化合物(10f)与非诺贝特一样有效。































 京公网安备 11010802027423号
京公网安备 11010802027423号