当前位置:
X-MOL 学术
›
Bioorgan. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Substituted phenyl[(5-benzyl-1,3,4-oxadiazol-2-yl)sulfanyl]acetates/acetamides as alkaline phosphatase inhibitors: Synthesis, computational studies, enzyme inhibitory kinetics and DNA binding studies.
Bioorganic Chemistry ( IF 4.5 ) Pub Date : 2019-07-03 , DOI: 10.1016/j.bioorg.2019.103108 Zafar Iqbal 1 , Zaman Ashraf 1 , Mubashir Hassan 2 , Qamar Abbas 3 , Erum Jabeen 1
Bioorganic Chemistry ( IF 4.5 ) Pub Date : 2019-07-03 , DOI: 10.1016/j.bioorg.2019.103108 Zafar Iqbal 1 , Zaman Ashraf 1 , Mubashir Hassan 2 , Qamar Abbas 3 , Erum Jabeen 1
Affiliation
|
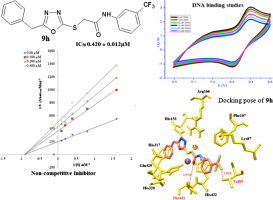
Substituted phenyl[(5-benzyl-1,3,4-oxadiazol-2-yl)sulfanyl]acetates/acetamides 9a-j were synthesized as alkaline phosphatase inhibitors. Phenyl acetic acid 1 through a series of reactions was converted into 5-benzyl-1,3,4-oxadiazole-2-thione 4. The intermediate oxadiazole 4 was then reacted with chloroacetyl derivatives of phenols 6a-f and anilines derivatives 8a-d to afford the title oxadiazole derivatives 9a-j. All of the title compounds 9a-j were evaluated for their inhibitory activity against human alkaline phosphatise (ALP). It was found that compounds 9a-j exhibited good to excellent alkaline phosphatase inhibitory activity especially 9h displayed potent activity with IC50 value 0.420 ± 0.012 µM while IC50 value of standard (KH2PO4) was 2.80 µM. The enzyme inhibitory kinetics of most potent inhibitor 9h was determined by Line-weaever Burk plots showing non-competitive mode of binding with enzyme. Molecular docking studies were performed against alkaline phosphatase enzyme (1EW2) to check the binding affinity of the synthesized compounds 9a-j against target protein. The compound 9h exhibited excellent binding affinity having binding energy value (-7.90 kcal/mol) compared to other derivatives. The brine shrimp viability assay results proved that derivative 9h was non-toxic at concentration used for enzyme assay. The lead compound 9h showed LD50 106.71 µM while the standard potassium dichromate showed LD50 0.891 µM. The DNA binding interactions of the synthesized compound 9h was also determined experimentally by spectrophotometric and electrochemical methods. The compound 9h was found to bind with grooves of DNA as depicted by both UV-Vis spectroscopy and cyclic voltammetry with binding constant values 7.83 × 103 and 7.95 × 103 M-1 respectively revealing significant strength of 9h-DNA complex. As dry lab and wet lab results concise each other it was concluded that synthesized compounds, especially compound 9h may serve as lead compound to design most potent inhibitors of human ALP.
中文翻译:

取代的苯基[(5-苄基-1,3,4-恶二唑-2-基)硫烷基]乙酸酯/乙酰胺为碱性磷酸酶抑制剂:合成,计算研究,酶抑制动力学和DNA结合研究。
合成了取代的苯基[(5-苄基-1,3,4-恶二唑-2-基)硫烷基]乙酸酯/乙酰胺9a-j,作为碱性磷酸酶抑制剂。通过一系列反应将苯乙酸1转化为5-苄基-1,3,4-恶二唑-2-硫酮4。然后使中间体恶二唑4与苯酚6a-f的氯乙酰基衍生物和苯胺衍生物8a-d反应得到标题的恶二唑衍生物9a-j。评价所有标题化合物9a-j对人碱性磷酸酶(ALP)的抑制活性。发现化合物9a-j表现出良好至优异的碱性磷酸酶抑制活性,尤其是9h显示出有效活性,IC50值为0.420±0.012μM,而标准品(KH2PO4)的IC50值为2.80μM。最有效的抑制剂9h的酶抑制动力学是通过Line-weaever Burk图确定的,该图显示了与酶结合的非竞争性模式。针对碱性磷酸酶(1EW2)进行了分子对接研究,以检查合成的化合物9a-j对靶蛋白的结合亲和力。与其他衍生物相比,化合物9h表现出优异的结合亲和力,具有结合能值(-7.90kcal / mol)。盐水虾的活力测定结果证明,衍生物9h在用于酶测定的浓度下是无毒的。铅化合物9h的LD50为106.71 µM,而标准重铬酸钾的LD50为0.891 µM。还通过分光光度法和电化学方法实验确定了合成化合物9h的DNA结合相互作用。如紫外-可见光谱和循环伏安法所示,发现化合物9h与DNA凹槽结合,结合常数分别为7.83×103和7.95×103 M-1,显示出9h-DNA复合物的显着强度。由于干实验室和湿实验室的结果相互简化,因此得出的结论是,合成的化合物(尤其是化合物9h)可以用作设计最有效的人类ALP抑制剂的先导化合物。
更新日期:2019-07-03
中文翻译:

取代的苯基[(5-苄基-1,3,4-恶二唑-2-基)硫烷基]乙酸酯/乙酰胺为碱性磷酸酶抑制剂:合成,计算研究,酶抑制动力学和DNA结合研究。
合成了取代的苯基[(5-苄基-1,3,4-恶二唑-2-基)硫烷基]乙酸酯/乙酰胺9a-j,作为碱性磷酸酶抑制剂。通过一系列反应将苯乙酸1转化为5-苄基-1,3,4-恶二唑-2-硫酮4。然后使中间体恶二唑4与苯酚6a-f的氯乙酰基衍生物和苯胺衍生物8a-d反应得到标题的恶二唑衍生物9a-j。评价所有标题化合物9a-j对人碱性磷酸酶(ALP)的抑制活性。发现化合物9a-j表现出良好至优异的碱性磷酸酶抑制活性,尤其是9h显示出有效活性,IC50值为0.420±0.012μM,而标准品(KH2PO4)的IC50值为2.80μM。最有效的抑制剂9h的酶抑制动力学是通过Line-weaever Burk图确定的,该图显示了与酶结合的非竞争性模式。针对碱性磷酸酶(1EW2)进行了分子对接研究,以检查合成的化合物9a-j对靶蛋白的结合亲和力。与其他衍生物相比,化合物9h表现出优异的结合亲和力,具有结合能值(-7.90kcal / mol)。盐水虾的活力测定结果证明,衍生物9h在用于酶测定的浓度下是无毒的。铅化合物9h的LD50为106.71 µM,而标准重铬酸钾的LD50为0.891 µM。还通过分光光度法和电化学方法实验确定了合成化合物9h的DNA结合相互作用。如紫外-可见光谱和循环伏安法所示,发现化合物9h与DNA凹槽结合,结合常数分别为7.83×103和7.95×103 M-1,显示出9h-DNA复合物的显着强度。由于干实验室和湿实验室的结果相互简化,因此得出的结论是,合成的化合物(尤其是化合物9h)可以用作设计最有效的人类ALP抑制剂的先导化合物。

































 京公网安备 11010802027423号
京公网安备 11010802027423号