当前位置:
X-MOL 学术
›
J. Chem. Eng. Data
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Solubility of 4-Chloro-2,5-dimethoxynitrobenzene, 4-Chloro-2,5-dimethoxyaniline, and 2,5-Dimethoxyaniline in Binary and Pure Solvents: Determination and Modeling
Journal of Chemical & Engineering Data ( IF 2.0 ) Pub Date : 2019-07-03 , DOI: 10.1021/acs.jced.9b00011 Xin Liu 1 , Bo Liu 1 , Yong-Sheng Xu 1 , Guo-Liang Zhang 1 , Qing Xia 1 , Feng-Bao Zhang 1
Journal of Chemical & Engineering Data ( IF 2.0 ) Pub Date : 2019-07-03 , DOI: 10.1021/acs.jced.9b00011 Xin Liu 1 , Bo Liu 1 , Yong-Sheng Xu 1 , Guo-Liang Zhang 1 , Qing Xia 1 , Feng-Bao Zhang 1
Affiliation
|
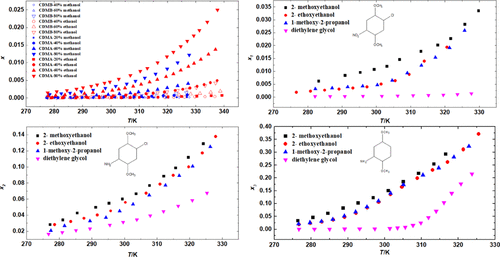
Using the dynamic method over the temperature range of (277 to 340) K at atmosphere pressure, solubility of 4-chloro-2,5-dimethoxynitrobenzene (CDMB) and 4-chloro-2,5-dimethoxyaniline (CDMA) in methanol–water and ethanol–water solvent mixtures, where solute-free mass fraction of methanol and ethanol range from 0.2 to 0.8, and solubility of CDMB, CDMA, and 2,5-dimethoxyaniline (DMAn) in 2-methoxyethanol, 2-ethoxyethanol, 1-methoxy-2-propanol, and diethylene glycol have been investigated. In all solvents, there exists a direct relationship between solubility and temperature. In mixture solvents, the experimental data are correlated using the Wilson and the NRTL models. The solubility of CDMB and CDMA increases with the added amount of methanol or ethanol. In pure organic solvents, significant differences are discerned for the solubility of CDMB, CDMA, and DMAn, and data are correlated with the Wilson, the NRTL, the modified Apelblat, and the λh models. The solubility of DMAn is strongly dependent on temperature, whereas CDMB is weakly dependent. On the basis of the solubility data and the solubility differences among CDMB, CDMA, and DMAn, ethanol–water solution is preferred to be the solvent for the reduction of CDMB, and diethylene glycol is suggested to be the recrystallization solvent used to separate DMAn from the system.
中文翻译:

4-氯-2,5-二甲氧基硝基苯,4-氯-2,5-二甲氧基苯胺和2,5-二甲氧基苯胺在二元和纯溶剂中的溶解度:测定和建模
在大气压力下使用动态方法在(277至340)K的温度范围内,将4-氯-2,5-二甲氧基硝基苯(CDMB)和4-氯-2,5-二甲氧基苯胺(CDMA)在甲醇-水中的溶解度和乙醇-水溶剂混合物,其中甲醇和乙醇的无溶质质量分数为0.2至0.8,CDMB,CDMA和2,5-二甲氧基苯胺(DMAn)在2-甲氧基乙醇,2-乙氧基乙醇,1-中的溶解度已经研究了甲氧基-2-丙醇和二甘醇。在所有溶剂中,溶解度和温度之间都存在直接关系。在混合溶剂中,使用Wilson和NRTL模型将实验数据关联起来。CDMB和CDMA的溶解度随甲醇或乙醇的添加而增加。在纯有机溶剂中,CDMB的溶解度存在明显差异,h模型。DMAn的溶解度强烈依赖于温度,而CDMB则弱依赖于温度。根据溶解度数据和CDMB,CDMA和DMAn之间的溶解度差异,乙醇水溶液最好是还原CDMB的溶剂,而建议使用二甘醇作为从中分离DMAn的重结晶溶剂。系统。
更新日期:2019-07-03
中文翻译:

4-氯-2,5-二甲氧基硝基苯,4-氯-2,5-二甲氧基苯胺和2,5-二甲氧基苯胺在二元和纯溶剂中的溶解度:测定和建模
在大气压力下使用动态方法在(277至340)K的温度范围内,将4-氯-2,5-二甲氧基硝基苯(CDMB)和4-氯-2,5-二甲氧基苯胺(CDMA)在甲醇-水中的溶解度和乙醇-水溶剂混合物,其中甲醇和乙醇的无溶质质量分数为0.2至0.8,CDMB,CDMA和2,5-二甲氧基苯胺(DMAn)在2-甲氧基乙醇,2-乙氧基乙醇,1-中的溶解度已经研究了甲氧基-2-丙醇和二甘醇。在所有溶剂中,溶解度和温度之间都存在直接关系。在混合溶剂中,使用Wilson和NRTL模型将实验数据关联起来。CDMB和CDMA的溶解度随甲醇或乙醇的添加而增加。在纯有机溶剂中,CDMB的溶解度存在明显差异,h模型。DMAn的溶解度强烈依赖于温度,而CDMB则弱依赖于温度。根据溶解度数据和CDMB,CDMA和DMAn之间的溶解度差异,乙醇水溶液最好是还原CDMB的溶剂,而建议使用二甘醇作为从中分离DMAn的重结晶溶剂。系统。































 京公网安备 11010802027423号
京公网安备 11010802027423号