Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Effect of Tanezumab on Joint Pain, Physical Function, and Patient Global Assessment of Osteoarthritis Among Patients With Osteoarthritis of the Hip or Knee
JAMA ( IF 63.1 ) Pub Date : 2019-07-02 , DOI: 10.1001/jama.2019.8044 Thomas J Schnitzer 1 , Richard Easton 2 , Shirley Pang 3 , Dennis J Levinson 4 , Glenn Pixton 5 , Lars Viktrup 6 , Isabelle Davignon 7 , Mark T Brown 7 , Christine R West 7 , Kenneth M Verburg 7
JAMA ( IF 63.1 ) Pub Date : 2019-07-02 , DOI: 10.1001/jama.2019.8044 Thomas J Schnitzer 1 , Richard Easton 2 , Shirley Pang 3 , Dennis J Levinson 4 , Glenn Pixton 5 , Lars Viktrup 6 , Isabelle Davignon 7 , Mark T Brown 7 , Christine R West 7 , Kenneth M Verburg 7
Affiliation
|
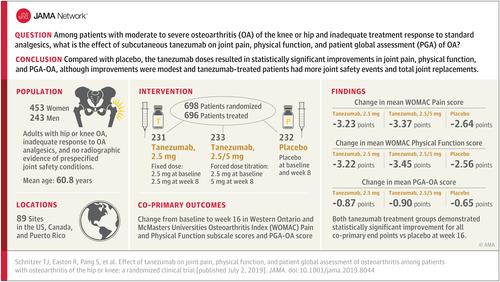
Importance
Patients with osteoarthritis (OA) may remain symptomatic with traditional OA treatments. Objective
To assess 2 subcutaneous tanezumab dosing regimens for OA. Design, Setting, and Participants
A randomized, double-blind, multicenter trial from January 2016 to May 14, 2018 (last patient visit). Patients enrolled were 18 years or older with hip or knee OA, inadequate response to OA analgesics, and no radiographic evidence of prespecified joint safety conditions. Interventions
Patients received by subcutaneous administration either tanezumab, 2.5 mg, at day 1 and week 8 (n = 231); tanezumab, 2.5 mg at day 1 and 5 mg at week 8 (ie, tanezumab, 2.5/5 mg; n = 233); or placebo at day 1 and week 8 (n = 232). Main Outcomes and Measures
Co-primary end points were change from baseline to week 16 in Western Ontario and McMasters Universities Osteoarthritis Index (WOMAC) Pain (0-10, no to extreme pain), WOMAC Physical Function (0-10, no to extreme difficulty), and patient global assessment of osteoarthritis (PGA-OA) (1-5, very good to very poor) scores. Results
Among 698 patients randomized, 696 received 1 or more treatment doses (mean [SD] age, 60.8 [9.6] years; 65.1% women), and 582 (83.6%) completed the trial. From baseline to 16 weeks, mean WOMAC Pain scores decreased from 7.1 to 3.6 in the tanezumab, 2.5 mg, group; 7.3 to 3.6 in the tanezumab, 2.5/5 mg, group; and 7.3 to 4.4 in the placebo group (least squares mean differences [95% CI] vs placebo were -0.60 [-1.07 to -0.13; P = .01] for tanezumab, 2.5 mg, and -0.73 [-1.20 to -0.26; P = .002] for tanezumab, 2.5/5 mg). Mean WOMAC Physical Function scores decreased from 7.2 to 3.7 in the 2.5-mg group, 7.4 to 3.6 in the 2.5/5-mg group, and 7.4 to 4.5 with placebo (differences vs placebo, -0.66 [-1.14 to -0.19; P = .007] for tanezumab, 2.5 mg, and -0.89 [-1.37 to -0.42; P < .001] for tanezumab, 2.5/5 mg). Mean PGA-OA scores decreased from 3.4 to 2.4 in the 2.5-mg group, 3.5 to 2.4 in the 2.5/5-mg group, and 3.5 to 2.7 with placebo (differences vs placebo, -0.22 [-0.39 to -0.05; P = .01] for tanezumab, 2.5 mg, and -0.25 [-0.41 to -0.08; P = .004] for tanezumab, 2.5/5 mg). Rapidly progressive OA occurred only in tanezumab-treated patients (2.5 mg: n = 5, 2.2%; 2.5/5 mg: n = 1, 0.4%). The incidence of total joint replacements was 8 (3.5%), 16 (6.9%), and 4 (1.7%) in the tanezumab, 2.5 mg; tanezumab, 2.5/5 mg; and placebo groups, respectively. Conclusions and Relevance
Among patients with moderate to severe OA of the knee or hip and inadequate response to standard analgesics, tanezumab, compared with placebo, resulted in statistically significant improvements in scores assessing pain and physical function, and in PGA-OA, although the improvements were modest and tanezumab-treated patients had more joint safety events and total joint replacements. Further research is needed to determine the clinical importance of these efficacy and adverse event findings. Trial Registration
ClinicalTrials.gov Identifier: NCT02697773.
中文翻译:

Tanezumab 对髋关节或膝关节骨关节炎患者的关节疼痛、身体功能和骨关节炎患者总体评估的影响
重要性 骨关节炎 (OA) 患者在接受传统 OA 治疗后可能仍会出现症状。目的评估 OA 的 2 种皮下 tanezumab 给药方案。设计、设置和参与者 2016 年 1 月至 2018 年 5 月 14 日(最后一次患者就诊)的随机、双盲、多中心试验。入组的患者年龄在 18 岁或以上,患有髋关节或膝关节 OA,对 OA 镇痛药的反应不足,并且没有预先指定的关节安全状况的放射学证据。干预 患者在第 1 天和第 8 周接受皮下注射 tanezumab,2.5 mg(n = 231);tanezumab,第 1 天 2.5 mg,第 8 周 5 mg(即,tanezumab,2.5/5 mg;n = 233);或安慰剂在第 1 天和第 8 周(n = 232)。主要结果和措施 共同主要终点是西安大略和麦克马斯特大学骨关节炎指数 (WOMAC) 疼痛(0-10,无至极度疼痛),WOMAC 身体功能(0-10,无至极度疼痛)从基线到第 16 周的变化难度),以及患者骨关节炎总体评估 (PGA-OA)(1-5,非常好到非常差)评分。结果 在随机分配的 698 名患者中,696 名接受了 1 个或更多的治疗剂量(平均 [SD] 年龄,60.8 [9.6] 岁;65.1% 为女性),582 名 (83.6%) 完成了试验。从基线到 16 周,tanezumab 2.5 mg 组的平均 WOMAC 疼痛评分从 7.1 降至 3.6;在 tanezumab 2.5/5 mg 组中为 7.3 至 3.6;安慰剂组为 7.3 至 4.4(tanezumab 2.5 mg 与安慰剂的最小二乘平均差异 [95% CI] 为 -0.60 [-1.07 至 -0.13;P = .01] 和 -0.73 [-1.20 至 -0.26 ; P = . 002] 为 tanezumab,2.5/5 毫克)。平均 WOMAC 身体功能评分在 2.5 毫克组中从 7.2 降至 3.7,在 2.5/5 毫克组中从 7.4 降至 3.6,安慰剂组从 7.4 降至 4.5(与安慰剂的差异,-0.66 [-1.14 至 -0.19;P = .007] 对于 tanezumab,2.5 mg,和 -0.89 [-1.37 至 -0.42;对于 tanezumab,P < .001],2.5/5 mg)。平均 PGA-OA 评分在 2.5 毫克组中从 3.4 降至 2.4,在 2.5/5 毫克组中从 3.5 降至 2.4,安慰剂组从 3.5 降至 2.7(与安慰剂的差异,-0.22 [-0.39 至 -0.05;P = .01],对于 tanezumab,2.5 mg,和 -0.25 [-0.41 至 -0.08;P = .004],对于 tanezumab,2.5/5 mg)。快速进展的 OA 仅发生在接受 tanezumab 治疗的患者中(2.5 mg:n = 5,2.2%;2.5/5 mg:n = 1,0.4%)。在 tanezumab 2.5 mg 中,全关节置换的发生率为 8 (3.5%)、16 (6.9%) 和 4 (1.7%);tanezumab,2.5/5 毫克;和安慰剂组,分别。结论和相关性 在膝关节或髋关节中度至重度 OA 且对标准镇痛剂反应不足的患者中,与安慰剂相比,tanezumab 导致疼痛和身体功能评估评分和 PGA-OA 评分有统计学显着改善,尽管有所改善中度和 tanezumab 治疗的患者有更多的关节安全事件和全关节置换。需要进一步的研究来确定这些疗效和不良事件发现的临床重要性。试验注册 ClinicalTrials.gov 标识符:NCT02697773。导致评估疼痛和身体功能的评分以及 PGA-OA 的评分有统计学显着改善,尽管改善不大,并且接受 tanezumab 治疗的患者有更多的关节安全事件和全关节置换术。需要进一步的研究来确定这些疗效和不良事件发现的临床重要性。试验注册 ClinicalTrials.gov 标识符:NCT02697773。导致评估疼痛和身体功能的评分以及 PGA-OA 的评分有统计学显着改善,尽管改善不大,并且接受 tanezumab 治疗的患者有更多的关节安全事件和全关节置换术。需要进一步的研究来确定这些疗效和不良事件发现的临床重要性。试验注册 ClinicalTrials.gov 标识符:NCT02697773。
更新日期:2019-07-02
中文翻译:

Tanezumab 对髋关节或膝关节骨关节炎患者的关节疼痛、身体功能和骨关节炎患者总体评估的影响
重要性 骨关节炎 (OA) 患者在接受传统 OA 治疗后可能仍会出现症状。目的评估 OA 的 2 种皮下 tanezumab 给药方案。设计、设置和参与者 2016 年 1 月至 2018 年 5 月 14 日(最后一次患者就诊)的随机、双盲、多中心试验。入组的患者年龄在 18 岁或以上,患有髋关节或膝关节 OA,对 OA 镇痛药的反应不足,并且没有预先指定的关节安全状况的放射学证据。干预 患者在第 1 天和第 8 周接受皮下注射 tanezumab,2.5 mg(n = 231);tanezumab,第 1 天 2.5 mg,第 8 周 5 mg(即,tanezumab,2.5/5 mg;n = 233);或安慰剂在第 1 天和第 8 周(n = 232)。主要结果和措施 共同主要终点是西安大略和麦克马斯特大学骨关节炎指数 (WOMAC) 疼痛(0-10,无至极度疼痛),WOMAC 身体功能(0-10,无至极度疼痛)从基线到第 16 周的变化难度),以及患者骨关节炎总体评估 (PGA-OA)(1-5,非常好到非常差)评分。结果 在随机分配的 698 名患者中,696 名接受了 1 个或更多的治疗剂量(平均 [SD] 年龄,60.8 [9.6] 岁;65.1% 为女性),582 名 (83.6%) 完成了试验。从基线到 16 周,tanezumab 2.5 mg 组的平均 WOMAC 疼痛评分从 7.1 降至 3.6;在 tanezumab 2.5/5 mg 组中为 7.3 至 3.6;安慰剂组为 7.3 至 4.4(tanezumab 2.5 mg 与安慰剂的最小二乘平均差异 [95% CI] 为 -0.60 [-1.07 至 -0.13;P = .01] 和 -0.73 [-1.20 至 -0.26 ; P = . 002] 为 tanezumab,2.5/5 毫克)。平均 WOMAC 身体功能评分在 2.5 毫克组中从 7.2 降至 3.7,在 2.5/5 毫克组中从 7.4 降至 3.6,安慰剂组从 7.4 降至 4.5(与安慰剂的差异,-0.66 [-1.14 至 -0.19;P = .007] 对于 tanezumab,2.5 mg,和 -0.89 [-1.37 至 -0.42;对于 tanezumab,P < .001],2.5/5 mg)。平均 PGA-OA 评分在 2.5 毫克组中从 3.4 降至 2.4,在 2.5/5 毫克组中从 3.5 降至 2.4,安慰剂组从 3.5 降至 2.7(与安慰剂的差异,-0.22 [-0.39 至 -0.05;P = .01],对于 tanezumab,2.5 mg,和 -0.25 [-0.41 至 -0.08;P = .004],对于 tanezumab,2.5/5 mg)。快速进展的 OA 仅发生在接受 tanezumab 治疗的患者中(2.5 mg:n = 5,2.2%;2.5/5 mg:n = 1,0.4%)。在 tanezumab 2.5 mg 中,全关节置换的发生率为 8 (3.5%)、16 (6.9%) 和 4 (1.7%);tanezumab,2.5/5 毫克;和安慰剂组,分别。结论和相关性 在膝关节或髋关节中度至重度 OA 且对标准镇痛剂反应不足的患者中,与安慰剂相比,tanezumab 导致疼痛和身体功能评估评分和 PGA-OA 评分有统计学显着改善,尽管有所改善中度和 tanezumab 治疗的患者有更多的关节安全事件和全关节置换。需要进一步的研究来确定这些疗效和不良事件发现的临床重要性。试验注册 ClinicalTrials.gov 标识符:NCT02697773。导致评估疼痛和身体功能的评分以及 PGA-OA 的评分有统计学显着改善,尽管改善不大,并且接受 tanezumab 治疗的患者有更多的关节安全事件和全关节置换术。需要进一步的研究来确定这些疗效和不良事件发现的临床重要性。试验注册 ClinicalTrials.gov 标识符:NCT02697773。导致评估疼痛和身体功能的评分以及 PGA-OA 的评分有统计学显着改善,尽管改善不大,并且接受 tanezumab 治疗的患者有更多的关节安全事件和全关节置换术。需要进一步的研究来确定这些疗效和不良事件发现的临床重要性。试验注册 ClinicalTrials.gov 标识符:NCT02697773。





















































 京公网安备 11010802027423号
京公网安备 11010802027423号