当前位置:
X-MOL 学术
›
Org. Process Res. Dev.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Cu-Catalyzed Couplings of Heteroaryl Primary Amines and (Hetero)aryl Bromides with 6-Hydroxypicolinamide Ligands
Organic Process Research & Development ( IF 3.1 ) Pub Date : 2019-07-02 00:00:00 , DOI: 10.1021/acs.oprd.9b00195 David J. Bernhardson 1 , Daniel W. Widlicka 1 , Robert A. Singer 1
Organic Process Research & Development ( IF 3.1 ) Pub Date : 2019-07-02 00:00:00 , DOI: 10.1021/acs.oprd.9b00195 David J. Bernhardson 1 , Daniel W. Widlicka 1 , Robert A. Singer 1
Affiliation
|
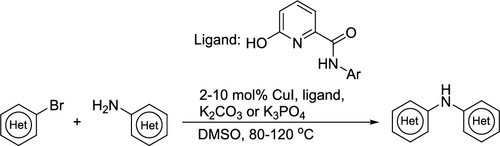
A family of 6-hydroxypicolinamide ligands have been identified as effective supporting ligands for Cu-catalyzed couplings of heteroaryl bromides and chlorides with heteroaryl primary amines. The C–N couplings are carried out at 80–120 °C in DMSO or sulfolane using K2CO3 or K3PO4 as the base with 2–10 mol % CuI and supporting ligand. The strength of the base was found to have an impact on the chemoselectivity and rate. The use of K2CO3 as the base enabled selective C–N coupling of aryl bromides over aryl chlorides with 2–5 mol % Cu at 80–120 °C. With K3PO4 as the base, aryl chlorides are capable of undergoing C–N coupling, though 5–10 mol % Cu is required at 120–130 °C. Members of the ligand family are straightforward to prepare in one step from 6-hydroxypicolinic acid and the corresponding anilines.
中文翻译:

铜催化杂芳基伯胺和(杂)芳基溴化物与6-羟基吡啶啉配体的偶联
已经确定了6-羟基吡啶甲酸酰胺配体家族是Cu催化的杂芳基溴化物和氯化物与杂芳基伯胺的偶联的有效支撑配体。C–N偶联是在80–120°C的DMSO或环丁砜中,以2-10 mol%CuI和支持配体的K 2 CO 3或K 3 PO 4为碱进行的。发现碱的强度对化学选择性和速率有影响。使用K 2 CO 3作为碱,可以在80–120°C的条件下,以2–5 mol%的Cu在芳基氯上进行芳基溴的选择性C–N偶联。含K 3 PO 4作为基础,芳基氯化物能够进行C–N偶联,尽管在120–130°C下需要5–10 mol%的Cu。配体家族的成员可以直接一步一步由6-羟基吡啶甲酸和相应的苯胺制备。
更新日期:2019-07-02
中文翻译:

铜催化杂芳基伯胺和(杂)芳基溴化物与6-羟基吡啶啉配体的偶联
已经确定了6-羟基吡啶甲酸酰胺配体家族是Cu催化的杂芳基溴化物和氯化物与杂芳基伯胺的偶联的有效支撑配体。C–N偶联是在80–120°C的DMSO或环丁砜中,以2-10 mol%CuI和支持配体的K 2 CO 3或K 3 PO 4为碱进行的。发现碱的强度对化学选择性和速率有影响。使用K 2 CO 3作为碱,可以在80–120°C的条件下,以2–5 mol%的Cu在芳基氯上进行芳基溴的选择性C–N偶联。含K 3 PO 4作为基础,芳基氯化物能够进行C–N偶联,尽管在120–130°C下需要5–10 mol%的Cu。配体家族的成员可以直接一步一步由6-羟基吡啶甲酸和相应的苯胺制备。















































 京公网安备 11010802027423号
京公网安备 11010802027423号