当前位置:
X-MOL 学术
›
Lancet Diabetes Endocrinol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Effects of alirocumab on cardiovascular and metabolic outcomes after acute coronary syndrome in patients with or without diabetes: a prespecified analysis of the ODYSSEY OUTCOMES randomised controlled trial.
The Lancet Diabetes & Endocrinology ( IF 44.0 ) Pub Date : 2019-07-01 , DOI: 10.1016/s2213-8587(19)30158-5 Kausik K Ray 1 , Helen M Colhoun 2 , Michael Szarek 3 , Marie Baccara-Dinet 4 , Deepak L Bhatt 5 , Vera A Bittner 6 , Andrzej J Budaj 7 , Rafael Diaz 8 , Shaun G Goodman 9 , Corinne Hanotin 10 , Robert A Harrington 11 , J Wouter Jukema 12 , Virginie Loizeau 10 , Renato D Lopes 13 , Angèle Moryusef 14 , Jan Murin 15 , Robert Pordy 16 , Arsen D Ristic 17 , Matthew T Roe 18 , José Tuñón 19 , Harvey D White 20 , Andreas M Zeiher 21 , Gregory G Schwartz 22 , Philippe Gabriel Steg 23 ,
The Lancet Diabetes & Endocrinology ( IF 44.0 ) Pub Date : 2019-07-01 , DOI: 10.1016/s2213-8587(19)30158-5 Kausik K Ray 1 , Helen M Colhoun 2 , Michael Szarek 3 , Marie Baccara-Dinet 4 , Deepak L Bhatt 5 , Vera A Bittner 6 , Andrzej J Budaj 7 , Rafael Diaz 8 , Shaun G Goodman 9 , Corinne Hanotin 10 , Robert A Harrington 11 , J Wouter Jukema 12 , Virginie Loizeau 10 , Renato D Lopes 13 , Angèle Moryusef 14 , Jan Murin 15 , Robert Pordy 16 , Arsen D Ristic 17 , Matthew T Roe 18 , José Tuñón 19 , Harvey D White 20 , Andreas M Zeiher 21 , Gregory G Schwartz 22 , Philippe Gabriel Steg 23 ,
Affiliation
|
BACKGROUND
After acute coronary syndrome, diabetes conveys an excess risk of ischaemic cardiovascular events. A reduction in mean LDL cholesterol to 1·4-1·8 mmol/L with ezetimibe or statins reduces cardiovascular events in patients with an acute coronary syndrome and diabetes. However, the efficacy and safety of further reduction in LDL cholesterol with an inhibitor of proprotein convertase subtilisin/kexin type 9 (PCSK9) after acute coronary syndrome is unknown. We aimed to explore this issue in a prespecified analysis of the ODYSSEY OUTCOMES trial of the PCSK9 inhibitor alirocumab, assessing its effects on cardiovascular outcomes by baseline glycaemic status, while also assessing its effects on glycaemic measures including risk of new-onset diabetes.
METHODS
ODYSSEY OUTCOMES was a randomised, double-blind, placebo-controlled trial, done at 1315 sites in 57 countries, that compared alirocumab with placebo in patients who had been admitted to hospital with an acute coronary syndrome (myocardial infarction or unstable angina) 1-12 months before randomisation and who had raised concentrations of atherogenic lipoproteins despite use of high-intensity statins. Patients were randomly assigned (1:1) to receive alirocumab or placebo every 2 weeks; randomisation was stratified by country and was done centrally with an interactive voice-response or web-response system. Alirocumab was titrated to target LDL cholesterol concentrations of 0·65-1·30 mmol/L. In this prespecified analysis, we investigated the effect of alirocumab on cardiovascular events by glycaemic status at baseline (diabetes, prediabetes, or normoglycaemia)-defined on the basis of patient history, review of medical records, or baseline HbA1c or fasting serum glucose-and risk of new-onset diabetes among those without diabetes at baseline. The primary endpoint was a composite of death from coronary heart disease, non-fatal myocardial infarction, fatal or non-fatal ischaemic stroke, or unstable angina requiring hospital admission. ODYSSEY OUTCOMES is registered with ClinicalTrials.gov, number NCT01663402.
FINDINGS
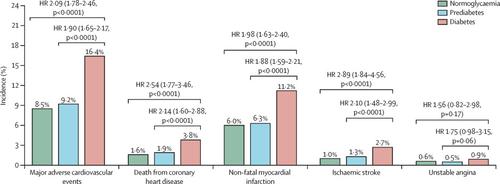
At study baseline, 5444 patients (28·8%) had diabetes, 8246 (43·6%) had prediabetes, and 5234 (27·7%) had normoglycaemia. There were no significant differences across glycaemic categories in median LDL cholesterol at baseline (2·20-2·28 mmol/L), after 4 months' treatment with alirocumab (0·80 mmol/L), or after 4 months' treatment with placebo (2·25-2·28 mmol/L). In the placebo group, the incidence of the primary endpoint over a median of 2·8 years was greater in patients with diabetes (16·4%) than in those with prediabetes (9·2%) or normoglycaemia (8·5%); hazard ratio (HR) for diabetes versus normoglycaemia 2·09 (95% CI 1·78-2·46, p<0·0001) and for diabetes versus prediabetes 1·90 (1·65-2·17, p<0·0001). Alirocumab resulted in similar relative reductions in the incidence of the primary endpoint in each glycaemic category, but a greater absolute reduction in the incidence of the primary endpoint in patients with diabetes (2·3%, 95% CI 0·4 to 4·2) than in those with prediabetes (1·2%, 0·0 to 2·4) or normoglycaemia (1·2%, -0·3 to 2·7; absolute risk reduction pinteraction=0·0019). Among patients without diabetes at baseline, 676 (10·1%) developed diabetes in the placebo group, compared with 648 (9·6%) in the alirocumab group; alirocumab did not increase the risk of new-onset diabetes (HR 1·00, 95% CI 0·89-1·11). HRs were 0·97 (95% CI 0·87-1·09) for patients with prediabetes and 1·30 (95% CI 0·93-1·81) for those with normoglycaemia (pinteraction=0·11).
INTERPRETATION
After a recent acute coronary syndrome, alirocumab treatment targeting an LDL cholesterol concentration of 0·65-1·30 mmol/L produced about twice the absolute reduction in cardiovascular events among patients with diabetes as in those without diabetes. Alirocumab treatment did not increase the risk of new-onset diabetes.
FUNDING
Sanofi and Regeneron Pharmaceuticals.
中文翻译:

Alirocumab对患有或不患有糖尿病的急性冠状动脉综合征后心血管和代谢结果的影响:对ODYSSEY结果随机对照试验的预先分析。
背景技术在急性冠状动脉综合征之后,糖尿病带来了缺血性心血管事件的过度风险。依泽替米贝或他汀类药物可将平均LDL胆固醇降低至1·4-1·8 mmol / L,可减少患有急性冠状动脉综合征和糖尿病的患者的心血管事件。然而,在急性冠状动脉综合征后用前蛋白转化酶枯草杆菌蛋白酶/ kexin 9型抑制剂(PCSK9)进一步降低LDL胆固醇的功效和安全性尚不清楚。我们旨在通过对PCSK9抑制剂alirocumab的ODYSSEY结果试验的预先分析来探讨此问题,通过基线血糖状态评估其对心血管预后的影响,同时还评估其对血糖测量的影响,包括新发糖尿病的风险。方法ODYSSEY结果是一项随机,双盲,安慰剂对照的试验,在57个国家/地区的1315个地点进行了这项研究,比较了在随机分配前1-12个月入院并患有急性冠状动脉综合征(心肌梗塞或不稳定型心绞痛)并且尽管使用了抗凝剂的患者中动脉粥样硬化脂蛋白浓度升高的患者中的alirocumab和安慰剂高强度他汀类药物。患者每2周随机分配(1:1)接受alirocumab或安慰剂治疗;随机化按国家/地区进行分层,并通过交互式语音响应或网络响应系统集中完成。Alirocumab的滴定目标LDL胆固醇浓度为0·65-1·30 mmol / L。在这项预先设定的分析中,我们调查了根据患者病史定义的基线(糖尿病,糖尿病前期或正常血糖)的血糖状态对阿洛洛单抗对心血管事件的影响,在基线时无糖尿病的患者中回顾病历或基线HbA1c或空腹血糖-以及新发糖尿病的风险。主要终点是因冠心病,非致命性心肌梗塞,致命或非致命性缺血性中风或需要住院的不稳定型心绞痛所致的死亡综合。ODYSSEY结果已在ClinicalTrials.gov注册,编号为NCT01663402。结果在研究基线时,有5444例患者(28·8%)患有糖尿病,有8246例(43·6%)患有糖尿病前期患者,有5234例(27·7%)患有常血糖症。在基线期(2·20-2·28 mmol / L),用阿罗昔单抗(0·80 mmol / L)治疗4个月或在血浆中LDL胆固醇治疗4个月后,各血糖类别的中位LDL胆固醇之间无显着差异。安慰剂(2·25-2·28 mmol / L)。在安慰剂组中,与糖尿病前期患者(9·2%)或血糖正常者(8·5%)相比,糖尿病患者(2·8年)主要终点的发生率更高(16·4%);糖尿病与正常血糖的危险比(HR)2·09(95%CI 1·78-2·46,p <0·0001),糖尿病与糖尿病前期的危险比1·90(1·65-2·17,p <0 ·0001)。Alirocumab导致每个血糖类别中主要终点发生率的相对降低相对相似,但糖尿病患者中主要终点出现率的绝对降低更大(2·3%,95%CI 0·4至4·2 )的比例高于糖尿病前期(1·2%,0·0至2·4)或血糖正常(1·2%,-0·3至2·7;降低绝对危险性的比率= 0·0019)的患者。在基线时无糖尿病的患者中,安慰剂组有676名(10·1%)患糖尿病,而alirocumab组则有648名(9·6%)。Alirocumab不会增加新发糖尿病的风险(HR 1·00,95%CI 0·89-1·11)。糖尿病前期患者的HRs为0·97(95%CI 0·87-1·09),正常血糖为1·30(95%CI 0·93-1·81)(交互作用= 0·11)。解释在最近的急性冠状动脉综合征之后,针对LDL胆固醇浓度为0·65-1·30 mmol / L的alirocumab治疗绝对降低了糖尿病患者的心血管事件,是无糖尿病患者的两倍。Alirocumab治疗并未增加新发糖尿病的风险。资助赛诺菲和再生元制药。解释在最近的急性冠状动脉综合征之后,针对LDL胆固醇浓度为0·65-1·30 mmol / L的alirocumab治疗绝对降低了糖尿病患者的心血管事件,是无糖尿病患者的两倍。Alirocumab治疗并未增加新发糖尿病的风险。资助赛诺菲和再生元制药。解释在最近的急性冠状动脉综合征之后,针对LDL胆固醇浓度为0·65-1·30 mmol / L的alirocumab治疗绝对降低了糖尿病患者的心血管事件,是无糖尿病患者的两倍。Alirocumab治疗并未增加新发糖尿病的风险。资助赛诺菲和再生元制药。
更新日期:2019-07-17
中文翻译:

Alirocumab对患有或不患有糖尿病的急性冠状动脉综合征后心血管和代谢结果的影响:对ODYSSEY结果随机对照试验的预先分析。
背景技术在急性冠状动脉综合征之后,糖尿病带来了缺血性心血管事件的过度风险。依泽替米贝或他汀类药物可将平均LDL胆固醇降低至1·4-1·8 mmol / L,可减少患有急性冠状动脉综合征和糖尿病的患者的心血管事件。然而,在急性冠状动脉综合征后用前蛋白转化酶枯草杆菌蛋白酶/ kexin 9型抑制剂(PCSK9)进一步降低LDL胆固醇的功效和安全性尚不清楚。我们旨在通过对PCSK9抑制剂alirocumab的ODYSSEY结果试验的预先分析来探讨此问题,通过基线血糖状态评估其对心血管预后的影响,同时还评估其对血糖测量的影响,包括新发糖尿病的风险。方法ODYSSEY结果是一项随机,双盲,安慰剂对照的试验,在57个国家/地区的1315个地点进行了这项研究,比较了在随机分配前1-12个月入院并患有急性冠状动脉综合征(心肌梗塞或不稳定型心绞痛)并且尽管使用了抗凝剂的患者中动脉粥样硬化脂蛋白浓度升高的患者中的alirocumab和安慰剂高强度他汀类药物。患者每2周随机分配(1:1)接受alirocumab或安慰剂治疗;随机化按国家/地区进行分层,并通过交互式语音响应或网络响应系统集中完成。Alirocumab的滴定目标LDL胆固醇浓度为0·65-1·30 mmol / L。在这项预先设定的分析中,我们调查了根据患者病史定义的基线(糖尿病,糖尿病前期或正常血糖)的血糖状态对阿洛洛单抗对心血管事件的影响,在基线时无糖尿病的患者中回顾病历或基线HbA1c或空腹血糖-以及新发糖尿病的风险。主要终点是因冠心病,非致命性心肌梗塞,致命或非致命性缺血性中风或需要住院的不稳定型心绞痛所致的死亡综合。ODYSSEY结果已在ClinicalTrials.gov注册,编号为NCT01663402。结果在研究基线时,有5444例患者(28·8%)患有糖尿病,有8246例(43·6%)患有糖尿病前期患者,有5234例(27·7%)患有常血糖症。在基线期(2·20-2·28 mmol / L),用阿罗昔单抗(0·80 mmol / L)治疗4个月或在血浆中LDL胆固醇治疗4个月后,各血糖类别的中位LDL胆固醇之间无显着差异。安慰剂(2·25-2·28 mmol / L)。在安慰剂组中,与糖尿病前期患者(9·2%)或血糖正常者(8·5%)相比,糖尿病患者(2·8年)主要终点的发生率更高(16·4%);糖尿病与正常血糖的危险比(HR)2·09(95%CI 1·78-2·46,p <0·0001),糖尿病与糖尿病前期的危险比1·90(1·65-2·17,p <0 ·0001)。Alirocumab导致每个血糖类别中主要终点发生率的相对降低相对相似,但糖尿病患者中主要终点出现率的绝对降低更大(2·3%,95%CI 0·4至4·2 )的比例高于糖尿病前期(1·2%,0·0至2·4)或血糖正常(1·2%,-0·3至2·7;降低绝对危险性的比率= 0·0019)的患者。在基线时无糖尿病的患者中,安慰剂组有676名(10·1%)患糖尿病,而alirocumab组则有648名(9·6%)。Alirocumab不会增加新发糖尿病的风险(HR 1·00,95%CI 0·89-1·11)。糖尿病前期患者的HRs为0·97(95%CI 0·87-1·09),正常血糖为1·30(95%CI 0·93-1·81)(交互作用= 0·11)。解释在最近的急性冠状动脉综合征之后,针对LDL胆固醇浓度为0·65-1·30 mmol / L的alirocumab治疗绝对降低了糖尿病患者的心血管事件,是无糖尿病患者的两倍。Alirocumab治疗并未增加新发糖尿病的风险。资助赛诺菲和再生元制药。解释在最近的急性冠状动脉综合征之后,针对LDL胆固醇浓度为0·65-1·30 mmol / L的alirocumab治疗绝对降低了糖尿病患者的心血管事件,是无糖尿病患者的两倍。Alirocumab治疗并未增加新发糖尿病的风险。资助赛诺菲和再生元制药。解释在最近的急性冠状动脉综合征之后,针对LDL胆固醇浓度为0·65-1·30 mmol / L的alirocumab治疗绝对降低了糖尿病患者的心血管事件,是无糖尿病患者的两倍。Alirocumab治疗并未增加新发糖尿病的风险。资助赛诺菲和再生元制药。












































 京公网安备 11010802027423号
京公网安备 11010802027423号