当前位置:
X-MOL 学术
›
Chem. Sci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
A reactive oxygen species-generating, cancer stem cell-potent manganese(ii) complex and its encapsulation into polymeric nanoparticles†
Chemical Science ( IF 7.6 ) Pub Date : 2019-07-02 00:00:00 , DOI: 10.1039/c9sc01275c Arvin Eskandari 1 , Kogularamanan Suntharalingam 2
Chemical Science ( IF 7.6 ) Pub Date : 2019-07-02 00:00:00 , DOI: 10.1039/c9sc01275c Arvin Eskandari 1 , Kogularamanan Suntharalingam 2
Affiliation
|
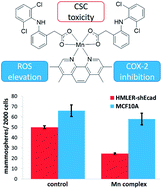
Intracellular redox modulation offers a viable approach to effectively remove cancer stem cells (CSCs), a subpopulation of tumour cells thought to be responsible for cancer recurrence and metastasis. Here we report the breast CSC potency of reactive oxygen species (ROS)-generating manganese(II)- and copper(II)-4,7-diphenyl-1,10-phenanthroline complexes bearing diclofenac, a nonsteriodial anti-inflammatory drug (NSAID), 1 and 3. Notably, the manganese(II) complex, 1, exhibits 9-fold, 31-fold, and 40-fold greater potency towards breast CSCs than 3, salinomycin (an established breast CSC-potent agent), and cisplatin (a clinically approved anticancer drug) respectively. Encouragingly, 1 displays 61-fold higher potency toward breast CSCs than normal skin fibroblast cells. Clinically relevant epithelial spheroid studies show that 1 is able to selectively inhibit breast CSC-enriched HMLER-shEcad mammosphere formation and viability (one order of magnitude) over non-tumorigenic breast MCF10A spheroids. Mechanistic studies show that 1 prompts breast CSC death by generating intracellular ROS and inhibiting cyclooxygenase-2 (COX-2) activity. The manganese(II) complex, 1, induces a greater degree of intracellular ROS in CSCs than the corresponding copper(II) complex, 3, highlighting the ROS-generating superiority of manganese(II)- over copper(II)-phenanthroline complexes. Encapsulation of 1 by biodegradable methoxy poly(ethylene glycol)-b-poly(D,L-lactic-co-glycolic) acid (PEG-PLGA) copolymers at the appropriate feed (5%, 1 NP5) enhances breast CSC uptake and greatly reduces overall toxicity. The nanoparticle formulation 1 NP5 indiscriminately kills breast CSCs and bulk breast cancer cells, and evokes a similar cellular response to the payload, 1. To the best of our knowledge, this is the first study to investigate the anti-CSC properties of managense complexes and to demonstrate that polymeric nanoparticles can be used to effectively deliver managense complexes into CSCs.
中文翻译:

一种产生活性氧、具有癌症干细胞活性的锰 (ii) 复合物及其封装在聚合物纳米颗粒中†
细胞内氧化还原调节提供了一种有效去除癌症干细胞(CSC)的可行方法,癌症干细胞是肿瘤细胞的一个亚群,被认为是导致癌症复发和转移的原因。在这里,我们报告了产生活性氧(ROS)的锰(II)-和铜(II)-4,7-二苯基-1,10-菲咯啉复合物与双氯芬酸(一种非甾体类抗炎药(NSAID))的乳腺CSC效力), 1和3。值得注意的是,锰 ( II ) 络合物1对乳腺 CSC 的效力比3、盐霉素(一种成熟的乳腺 CSC 有效药物)和顺铂(一种临床批准的抗癌药物)高9 倍、31 倍和 40 倍药)分别。令人鼓舞的是,1对乳腺 CSC 的效力比正常皮肤成纤维细胞高 61 倍。临床相关的上皮球体研究表明,与非致瘤性乳腺 MCF10A 球体相比, 1能够选择性抑制乳腺 CSC 富集的 HMLER-shEcad 乳腺球体的形成和活力(一个数量级)。机制研究表明1通过产生细胞内 ROS 和抑制环氧合酶-2 (COX-2) 活性来促进乳腺 CSC 死亡。锰 ( II ) 络合物1比相应的铜 ( II ) 络合物3在 CSC 中诱导更大程度的细胞内 ROS,这凸显了锰 ( II )- 相对于铜 ( II )-菲咯啉络合物在ROS 生成方面的优势。在适当的饲料(5%, 1 NP 5 )中,通过可生物降解的甲氧基聚(乙二醇)-b-聚(D,L-乳酸-共-乙醇)酸(PEG-PLGA)共聚物封装1可增强乳腺CSC的吸收和大大降低了总体毒性。纳米颗粒制剂1 NP 5不加区别地杀死乳腺 CSC 和大量乳腺癌细胞,并引起对有效负载1的类似细胞反应。据我们所知,这是第一项研究锰矿复合物的抗 CSC 特性并证明聚合物纳米颗粒可用于有效地将锰矿复合物递送至 CSC 中。
更新日期:2019-07-02
中文翻译:

一种产生活性氧、具有癌症干细胞活性的锰 (ii) 复合物及其封装在聚合物纳米颗粒中†
细胞内氧化还原调节提供了一种有效去除癌症干细胞(CSC)的可行方法,癌症干细胞是肿瘤细胞的一个亚群,被认为是导致癌症复发和转移的原因。在这里,我们报告了产生活性氧(ROS)的锰(II)-和铜(II)-4,7-二苯基-1,10-菲咯啉复合物与双氯芬酸(一种非甾体类抗炎药(NSAID))的乳腺CSC效力), 1和3。值得注意的是,锰 ( II ) 络合物1对乳腺 CSC 的效力比3、盐霉素(一种成熟的乳腺 CSC 有效药物)和顺铂(一种临床批准的抗癌药物)高9 倍、31 倍和 40 倍药)分别。令人鼓舞的是,1对乳腺 CSC 的效力比正常皮肤成纤维细胞高 61 倍。临床相关的上皮球体研究表明,与非致瘤性乳腺 MCF10A 球体相比, 1能够选择性抑制乳腺 CSC 富集的 HMLER-shEcad 乳腺球体的形成和活力(一个数量级)。机制研究表明1通过产生细胞内 ROS 和抑制环氧合酶-2 (COX-2) 活性来促进乳腺 CSC 死亡。锰 ( II ) 络合物1比相应的铜 ( II ) 络合物3在 CSC 中诱导更大程度的细胞内 ROS,这凸显了锰 ( II )- 相对于铜 ( II )-菲咯啉络合物在ROS 生成方面的优势。在适当的饲料(5%, 1 NP 5 )中,通过可生物降解的甲氧基聚(乙二醇)-b-聚(D,L-乳酸-共-乙醇)酸(PEG-PLGA)共聚物封装1可增强乳腺CSC的吸收和大大降低了总体毒性。纳米颗粒制剂1 NP 5不加区别地杀死乳腺 CSC 和大量乳腺癌细胞,并引起对有效负载1的类似细胞反应。据我们所知,这是第一项研究锰矿复合物的抗 CSC 特性并证明聚合物纳米颗粒可用于有效地将锰矿复合物递送至 CSC 中。











































 京公网安备 11010802027423号
京公网安备 11010802027423号