当前位置:
X-MOL 学术
›
Nano Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Silicon Carbide as a Protective Layer to Stabilize Si-Based Anodes by Inhibiting Chemical Reactions
Nano Letters ( IF 9.6 ) Pub Date : 2019-07-01 00:00:00 , DOI: 10.1021/acs.nanolett.9b01492 Chunhui Yu 1 , Xiao Chen 1 , Zhexi Xiao 1 , Chao Lei 1 , Chenxi Zhang 1 , Xianqing Lin 1 , Boyuan Shen 1 , Rufan Zhang 1 , Fei Wei 1
Nano Letters ( IF 9.6 ) Pub Date : 2019-07-01 00:00:00 , DOI: 10.1021/acs.nanolett.9b01492 Chunhui Yu 1 , Xiao Chen 1 , Zhexi Xiao 1 , Chao Lei 1 , Chenxi Zhang 1 , Xianqing Lin 1 , Boyuan Shen 1 , Rufan Zhang 1 , Fei Wei 1
Affiliation
|
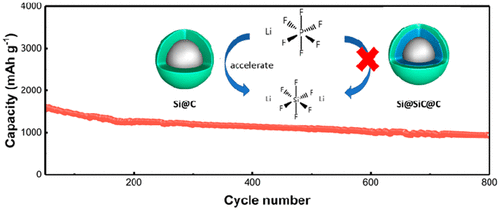
Developing a practical silicon-based (Si-based) anode is a precondition for high-performance lithium-ion batteries. However, the chemical reactivity of the Si renders it liable to be consumed, which must be completely understood for it to be used in practical battery systems. Here, a fresh and fundamental mechanism is proposed for the rapid failure of Si-based materials. Silicon can chemically react with lithium hexafluorophosphate (LiPF6) to constantly generate lithium hexafluorosilicate (Li2SiF6) aggregates during cycling. In addition, nanocarbon coated on silicon acts as a catalyst to accelerate such detrimental reactions. By taking advantage of the high strength and toughness of silicon carbide (SiC), a SiC layer is introduced between the inner silicon and outer carbon layers to inhibit the formation of Li2SiF6. The side reaction rate decreases significantly due to the increase in the activation energy of the reaction. [email protected]@C maintains a specific capacity of 980 mAh g–1 at a current density of 1 A g–1 after 800 cycles with an initial Coulombic efficiency over 88.5%. This study will contribute to improved design of Si-based anode for high-performance Li-ion batteries.
中文翻译:

碳化硅作为保护层,通过抑制化学反应来稳定硅基阳极
开发实用的硅基(Si基)阳极是高性能锂离子电池的前提。然而,Si的化学反应性使其易于被消耗,必须将其完全理解才能用于实际的电池系统中。在此,提出了一种用于硅基材料快速失效的新的基本机制。硅可以与六氟磷酸锂(LiPF 6)化学反应以不断生成六氟硅酸锂(Li 2 SiF 6)在循环过程中聚集。另外,涂覆在硅上的纳米碳充当催化剂以加速这种有害反应。通过利用碳化硅(SiC)的高强度和韧性,在内硅层和外碳层之间引入SiC层以抑制Li 2 SiF 6的形成。由于反应活化能的增加,副反应速率显着降低。[电子邮件保护] @C经过800次循环后,在1 A g –1的电流密度下可保持980 mAh g –1的比容量,初始库仑效率超过88.5%。这项研究将有助于改进高性能锂离子电池硅基负极的设计。
更新日期:2019-07-01
中文翻译:

碳化硅作为保护层,通过抑制化学反应来稳定硅基阳极
开发实用的硅基(Si基)阳极是高性能锂离子电池的前提。然而,Si的化学反应性使其易于被消耗,必须将其完全理解才能用于实际的电池系统中。在此,提出了一种用于硅基材料快速失效的新的基本机制。硅可以与六氟磷酸锂(LiPF 6)化学反应以不断生成六氟硅酸锂(Li 2 SiF 6)在循环过程中聚集。另外,涂覆在硅上的纳米碳充当催化剂以加速这种有害反应。通过利用碳化硅(SiC)的高强度和韧性,在内硅层和外碳层之间引入SiC层以抑制Li 2 SiF 6的形成。由于反应活化能的增加,副反应速率显着降低。[电子邮件保护] @C经过800次循环后,在1 A g –1的电流密度下可保持980 mAh g –1的比容量,初始库仑效率超过88.5%。这项研究将有助于改进高性能锂离子电池硅基负极的设计。































 京公网安备 11010802027423号
京公网安备 11010802027423号