当前位置:
X-MOL 学术
›
Chem. Eur. J.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Mechanisms in Iodine Catalysis
Chemistry - A European Journal ( IF 3.9 ) Pub Date : 2018-04-30 , DOI: 10.1002/chem.201706136 Martin Breugst 1 , Daniel von der Heiden 1
Chemistry - A European Journal ( IF 3.9 ) Pub Date : 2018-04-30 , DOI: 10.1002/chem.201706136 Martin Breugst 1 , Daniel von der Heiden 1
Affiliation
|
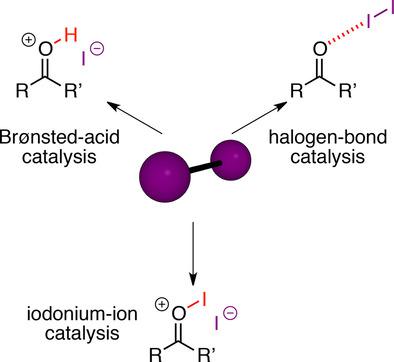
Molecular iodine has been used for more than 100 years as a remarkable catalyst for many organic transformations such as cycloadditions, Michael and aldol reactions, or esterifications. Different explanations for the origin of its catalytic effect have been proposed in the last decades including a “hidden” Brønsted acid catalysis by HI, a Lewis‐acid (or halogen‐bond) activation, or catalysis by an iodonium(I) species. Recently, iodine catalysis again gained more interest due to the latest developments in halogen‐bond catalysis. In this Minireview, we first summarize the experimental basis for the proposed modes of activation. Subsequently, we analyze typical iodine‐catalyzed reactions to gain more insights into the underlying reaction mechanisms.
中文翻译:

碘催化的机理
分子碘作为许多有机转化(例如环加成反应,迈克尔和羟醛反应或酯化反应)的杰出催化剂已使用了100多年。在过去的几十年中,人们对其催化作用的起源提出了不同的解释,包括HI的“隐式”布朗斯台德酸催化,路易斯酸(或卤素键)活化或碘鎓(I)物种的催化作用。最近,由于卤素键催化的最新进展,碘催化再次引起了人们的兴趣。在此Minireview中,我们首先总结了提出的激活模式的实验基础。随后,我们分析了典型的碘催化反应,以深入了解潜在的反应机理。
更新日期:2018-04-30
中文翻译:

碘催化的机理
分子碘作为许多有机转化(例如环加成反应,迈克尔和羟醛反应或酯化反应)的杰出催化剂已使用了100多年。在过去的几十年中,人们对其催化作用的起源提出了不同的解释,包括HI的“隐式”布朗斯台德酸催化,路易斯酸(或卤素键)活化或碘鎓(I)物种的催化作用。最近,由于卤素键催化的最新进展,碘催化再次引起了人们的兴趣。在此Minireview中,我们首先总结了提出的激活模式的实验基础。随后,我们分析了典型的碘催化反应,以深入了解潜在的反应机理。





















































 京公网安备 11010802027423号
京公网安备 11010802027423号