当前位置:
X-MOL 学术
›
Angew. Chem. Int. Ed.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Rational Tuning of Fluorobenzene Probes for Cysteine‐Selective Protein Modification
Angewandte Chemie International Edition ( IF 16.1 ) Pub Date : 2018-04-14 , DOI: 10.1002/anie.201712589 Ahmed M. Embaby 1 , Sanne Schoffelen 1 , Christian Kofoed 1 , Morten Meldal 1 , Frederik Diness 1
Angewandte Chemie International Edition ( IF 16.1 ) Pub Date : 2018-04-14 , DOI: 10.1002/anie.201712589 Ahmed M. Embaby 1 , Sanne Schoffelen 1 , Christian Kofoed 1 , Morten Meldal 1 , Frederik Diness 1
Affiliation
|
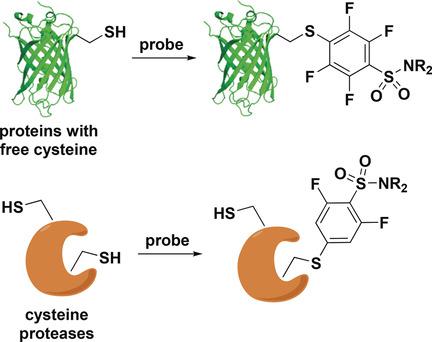
Fluorobenzene probes for protein profiling through selective cysteine labeling have been developed by rational reactivity tuning. Tuning was achieved by selecting an electron‐withdrawing para substituent in combination with variation of the number of fluorine substituents. Optimized probes chemoselectively arylated cysteine residues in proteins under aqueous conditions. Probes linked to azide, biotin, or a fluorophore were applicable to labeling of eGFP and albumin. Selective inhibition of cysteine proteases was also demonstrated with the probes. Additionally, probes were tuned for site‐selective labeling of cysteine residues and for activity‐based protein profiling in cell lysates.
中文翻译:

半胱氨酸选择性蛋白修饰的氟苯探针的合理调整。
通过合理的反应性调节,已经开发出用于通过选择性半胱氨酸标记进行蛋白质谱分析的氟苯探针。通过选择吸电子对位取代基并结合氟取代基数量的变化来实现调谐。优化的探针在水性条件下化学选择性芳基化蛋白质中的半胱氨酸残基。与叠氮化物,生物素或荧光团连接的探针可用于标记eGFP和白蛋白。探针也证明了对半胱氨酸蛋白酶的选择性抑制。此外,还对探针进行了调整,以对半胱氨酸残基进行位点选择性标记,并针对细胞裂解液中基于活性的蛋白质谱进行了分析。
更新日期:2018-04-14
中文翻译:

半胱氨酸选择性蛋白修饰的氟苯探针的合理调整。
通过合理的反应性调节,已经开发出用于通过选择性半胱氨酸标记进行蛋白质谱分析的氟苯探针。通过选择吸电子对位取代基并结合氟取代基数量的变化来实现调谐。优化的探针在水性条件下化学选择性芳基化蛋白质中的半胱氨酸残基。与叠氮化物,生物素或荧光团连接的探针可用于标记eGFP和白蛋白。探针也证明了对半胱氨酸蛋白酶的选择性抑制。此外,还对探针进行了调整,以对半胱氨酸残基进行位点选择性标记,并针对细胞裂解液中基于活性的蛋白质谱进行了分析。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号