European Journal of Medicinal Chemistry ( IF 6.0 ) Pub Date : 2019-07-01 , DOI: 10.1016/j.ejmech.2019.06.088 Divan G. van Greunen , C. Johan van der Westhuizen , Werner Cordier , Margo Nell , Andre Stander , Vanessa Steenkamp , Jenny-Lee Panayides , Darren L. Riley
|
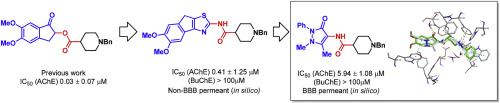
A series of fifteen acetylcholinesterase inhibitors were designed and synthesised based upon the previously identified lead compound 5,6-dimethoxy-1-oxo-2,3-dihydro-1H-inden-2-yl 1-benzylpiperidine-4-carboxylate (5) which showed good inhibitory activity (IC50 0.03 ± 0.07 μM) against acetylcholinesterase. A series of compounds were prepared wherein the ester linker in the original lead compound was exchanged for a more metabolically stable amide linker and the indanone moiety was exchanged for a range of aryl and aromatic heterocycles. The two most active analogues 1-benzyl-N-(5,6-dimethoxy-8H-indeno[1,2-d]thiazol-2-yl)piperidine-4-carboxamide (28) and 1-benzyl-N-(1-methyl-3-oxo-2-phenyl-2,3-dihydro-1H-pyrazol-4-yl) piperidine-4-carboxamide (20) afforded in vitro IC50 values of 0.41 ± 1.25 and 5.94 ± 1.08 μM, respectively. In silico screening predicts that 20 will be a blood brain-barrier permeant, and molecular dynamic simulations are indicative of a close correlation between the binding of 20 and the Food and Drug Administration-approved cholinesterase inhibitor donepezil (1).
中文翻译:

新型N-苄基哌啶羧酰胺衍生物可作为潜在的胆碱酯酶抑制剂治疗阿尔茨海默氏病
根据先前确定的先导化合物5,6-二甲氧基-1-氧代-2,3-二氢-1H-茚满-2-基-1-苄基哌啶-4-羧酸酯设计并合成了十五种乙酰胆碱酯酶抑制剂系列(5)对乙酰胆碱酯酶显示出良好的抑制活性(IC 50 0.03±0.07μM)。制备了一系列化合物,其中将原始先导化合物中的酯连接基交换为代谢上更稳定的酰胺连接基,并将茚满酮部分交换为一系列芳基和芳族杂环。两个最活性类似物1-苄基- ñ - (5,6-二甲基-8H-茚并[1,2-d]噻唑-2-基)哌啶-4-甲酰胺(28)和1-苄基Ñ-(1-甲基-3-氧代-2-苯基-2,3-二氢-1H-吡唑-4-基)哌啶-4-甲酰胺(20)的体外IC 50值为0.41±1.25和5.94±1.08分别为μM。在计算机筛查中预测20将成为血脑屏障渗透物,分子动力学模拟表明20的结合与美国食品药品监督管理局批准的胆碱酯酶抑制剂多奈哌齐(1)紧密相关。

































 京公网安备 11010802027423号
京公网安备 11010802027423号