Communications Chemistry ( IF 5.9 ) Pub Date : 2019-06-28 , DOI: 10.1038/s42004-019-0174-8
Dongwei Kang , Tong Zhao , Zhao Wang , Da Feng , Heng Zhang , Boshi Huang , Gaochan Wu , Fenju Wei , Zhongxia Zhou , Lanlan Jing , Xiaofang Zuo , Ye Tian , Vasanthanathan Poongavanam , Jacob Kongsted , Erik De Clercq , Christophe Pannecouque , Peng Zhan , Xinyong Liu
|
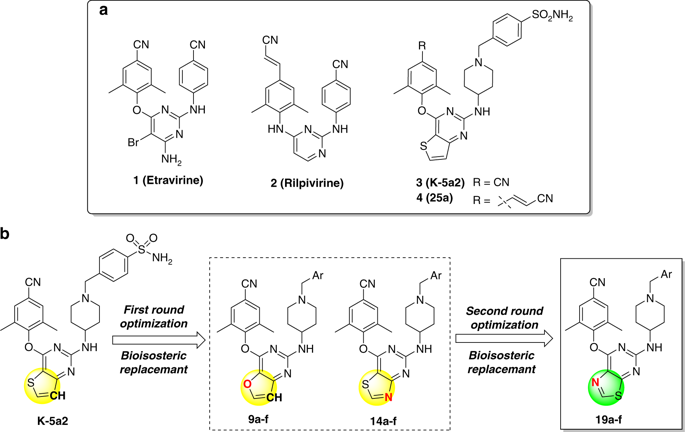
HIV-1 reverse transcriptase offers a key target for antiviral therapy. However, the rapid emergence of drug-resistant mutations in reverse transcriptase as well as the poor pharmacokinetic properties of HIV-1 non-nucleoside reverse transcriptase inhibitors (NNRTIs) limits their clinical use. Starting from a previous piperidine-substituted thiophene[3,2-d]pyrimidine compound (K-5a2), here we explore the chemical space around the thiophene ring located in the solvent-exposed regions of the NNRTI binding pocket in detail. Bioisosterism-based structural modification leads to the discovery of a number of compounds as potent in vitro reverse transcriptase inhibitors, providing improved drug resistance profiles compared to the listed drug Etravirine. Furthermore, 14a and 19a are identified as lead compounds with good solubility, appropriate ligand efficiency, and lower cytochrome P450 liability. Compound 19a exhibits useful in vivo pharmacokinetic properties in rat and safety in mice, suggesting that it may have the potential to be an effective drug candidate for treating AIDS.
中文翻译:

发现哌啶取代的噻唑并[5,4-d]嘧啶衍生物作为有效的和口服可生物利用的HIV-1非核苷逆转录酶抑制剂
HIV-1逆转录酶是抗病毒治疗的关键靶标。但是,逆转录酶中耐药突变的迅速出现以及HIV-1非核苷逆转录酶抑制剂(NNRTIs)的不良药代动力学特性限制了其临床应用。从以前的哌啶取代的噻吩[3,2- d ]嘧啶化合物(K-5a2)开始,在这里我们详细研究了位于NNRTI结合口袋中溶剂暴露区域的噻吩环周围的化学空间。基于生物立体异构的结构修饰导致发现许多化合物作为有效的体外逆转录酶抑制剂,与列出的药物依特韦林相比,具有更好的耐药性。此外,14a和19a被鉴定为具有良好溶解性,合适的配体效率和较低的细胞色素P450耐受性的先导化合物。化合物19a在大鼠中显示出有用的体内药代动力学性质,在小鼠中显示出安全性,表明它可能具有成为治疗艾滋病的有效候选药物的潜力。

































 京公网安备 11010802027423号
京公网安备 11010802027423号