当前位置:
X-MOL 学术
›
ACS Appl. Mater. Interfaces
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Azoreductase-Responsive Metal-Organic Framework-Based Nanodrug for Enhanced Cancer Therapy via Breaking Hypoxia-induced Chemoresistance.
ACS Applied Materials & Interfaces ( IF 8.3 ) Pub Date : 2019-07-10 , DOI: 10.1021/acsami.9b08115 Caixia Huang 1 , Wenlong Tan 1 , Jing Zheng 1 , Cong Zhu 1 , Jia Huo 1, 2 , Ronghua Yang 3
ACS Applied Materials & Interfaces ( IF 8.3 ) Pub Date : 2019-07-10 , DOI: 10.1021/acsami.9b08115 Caixia Huang 1 , Wenlong Tan 1 , Jing Zheng 1 , Cong Zhu 1 , Jia Huo 1, 2 , Ronghua Yang 3
Affiliation
|
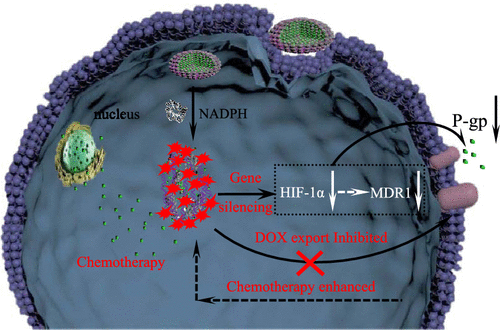
The insufficient oxygen supply may cause hypoxia in a solid tumor, which can lead to drug resistance and unsatisfactory chemotherapy effect. To address this issue, a new nanodrug has been developed with azoreductase-responsive functional metal-organic frameworks (AMOFs), where chemotherapeutic drugs were encapsulated in the AMOFs and small interfering RNAs (siRNAs) were absorbed on the surface of AMOFs. The siRNA was designed to contain hypoxia-inducible factor (HIF)-1α against RX-0047, which can induce significant downregulation of HIF-1α protein. The azobenzene units within the frameworks of AMOFs could be reduced to amines by the highly expressed azoreductase under the oxygen-deficient environment, which results in azoreductase-responsive release of the encapsulated drugs and siRNAs under the hypoxic condition. Therefore, once the drug-loaded AMOF entered the hypoxic cancer cells, the azoreductase-responsive release of siRNA could decrease the efflux of chemotherapeutic drugs via inhibiting the expressions of HIF-1α, multidrug resistance gene 1, and P-glycoprotein. This nanodrug can thus efficiently break hypoxia-induced chemoresistance and result in high-efficient cancer therapy in hypoxic tumors. As far as we know, this is the first attempt to construct an AMOF-based nanodrug with hypoxic harvesting behaviors. This proof-of-concept research provides a simple strategy for the construction of hypoxic-responsive AMOFs and also offers a unique on-command drug delivery platform, which can effectively break hypoxia-induced chemoresistance.
中文翻译:

偶氮还原酶反应性金属有机骨架为基础的纳米药物通过打破缺氧诱导的化学抗性增强癌症治疗。
氧气供应不足可能会导致实体瘤缺氧,从而导致耐药性和化疗效果不理想。为了解决这个问题,已经开发了一种具有偶氮还原酶反应性功能金属有机框架(AMOF)的新型纳米药物,其中化疗药物被封装在AMOF中,小的干扰RNA(siRNA)被吸收在AMOF的表面上。siRNA被设计为包含针对RX-0047的缺氧诱导因子(HIF)-1α,它可以诱导HIF-1α蛋白的显着下调。在缺氧条件下,高表达的偶氮还原酶可将AMOFs框架内的偶氮苯单元还原为胺,从而在缺氧条件下导致封装药物和siRNA的偶氮还原酶反应性释放。所以,一旦载药的AMOF进入低氧癌细胞,siRNA的偶氮还原酶响应释放可能会通过抑制HIF-1α,多药耐药基因1和P-糖蛋白的表达而降低化疗药物的流出。因此,这种纳米药物可以有效地打破低氧诱导的化学耐药性,并在缺氧肿瘤中导致高效的癌症治疗。据我们所知,这是构建具有缺氧收获行为的基于AMOF的纳米药物的首次尝试。这项概念验证研究为构建低氧反应性AMOF提供了一种简单策略,并且还提供了一个独特的按需给药平台,可以有效打破低氧诱导的化学耐药性。siRNA的偶氮还原酶反应性释放可通过抑制HIF-1α,多药耐药基因1和P-糖蛋白的表达而降低化疗药物的流出。因此,这种纳米药物可以有效地打破缺氧诱导的化学耐药性,并在缺氧肿瘤中导致高效的癌症治疗。据我们所知,这是构建具有缺氧收获行为的基于AMOF的纳米药物的首次尝试。这项概念验证研究为构建低氧反应性AMOF提供了一种简单策略,并且还提供了一个独特的按需给药平台,可以有效打破低氧诱导的化学耐药性。siRNA的偶氮还原酶反应性释放可通过抑制HIF-1α,多药耐药基因1和P-糖蛋白的表达而降低化疗药物的流出。因此,这种纳米药物可以有效地打破低氧诱导的化学耐药性,并在缺氧肿瘤中导致高效的癌症治疗。据我们所知,这是构建具有缺氧收获行为的基于AMOF的纳米药物的首次尝试。这项概念验证研究为构建低氧反应性AMOF提供了一种简单策略,并且还提供了一个独特的按需给药平台,可以有效地打破低氧诱导的化学抗药性。因此,这种纳米药物可以有效地打破低氧诱导的化学耐药性,并在缺氧肿瘤中导致高效的癌症治疗。据我们所知,这是构建具有缺氧收获行为的基于AMOF的纳米药物的首次尝试。这项概念验证研究为构建低氧反应性AMOF提供了一种简单策略,并且还提供了一个独特的按需给药平台,可以有效打破低氧诱导的化学耐药性。因此,这种纳米药物可以有效地打破低氧诱导的化学耐药性,并在缺氧肿瘤中导致高效的癌症治疗。据我们所知,这是构建具有缺氧收获行为的基于AMOF的纳米药物的首次尝试。这项概念验证研究为构建低氧反应性AMOF提供了一种简单策略,并且还提供了一个独特的按需给药平台,可以有效打破低氧诱导的化学耐药性。
更新日期:2019-06-28
中文翻译:

偶氮还原酶反应性金属有机骨架为基础的纳米药物通过打破缺氧诱导的化学抗性增强癌症治疗。
氧气供应不足可能会导致实体瘤缺氧,从而导致耐药性和化疗效果不理想。为了解决这个问题,已经开发了一种具有偶氮还原酶反应性功能金属有机框架(AMOF)的新型纳米药物,其中化疗药物被封装在AMOF中,小的干扰RNA(siRNA)被吸收在AMOF的表面上。siRNA被设计为包含针对RX-0047的缺氧诱导因子(HIF)-1α,它可以诱导HIF-1α蛋白的显着下调。在缺氧条件下,高表达的偶氮还原酶可将AMOFs框架内的偶氮苯单元还原为胺,从而在缺氧条件下导致封装药物和siRNA的偶氮还原酶反应性释放。所以,一旦载药的AMOF进入低氧癌细胞,siRNA的偶氮还原酶响应释放可能会通过抑制HIF-1α,多药耐药基因1和P-糖蛋白的表达而降低化疗药物的流出。因此,这种纳米药物可以有效地打破低氧诱导的化学耐药性,并在缺氧肿瘤中导致高效的癌症治疗。据我们所知,这是构建具有缺氧收获行为的基于AMOF的纳米药物的首次尝试。这项概念验证研究为构建低氧反应性AMOF提供了一种简单策略,并且还提供了一个独特的按需给药平台,可以有效打破低氧诱导的化学耐药性。siRNA的偶氮还原酶反应性释放可通过抑制HIF-1α,多药耐药基因1和P-糖蛋白的表达而降低化疗药物的流出。因此,这种纳米药物可以有效地打破缺氧诱导的化学耐药性,并在缺氧肿瘤中导致高效的癌症治疗。据我们所知,这是构建具有缺氧收获行为的基于AMOF的纳米药物的首次尝试。这项概念验证研究为构建低氧反应性AMOF提供了一种简单策略,并且还提供了一个独特的按需给药平台,可以有效打破低氧诱导的化学耐药性。siRNA的偶氮还原酶反应性释放可通过抑制HIF-1α,多药耐药基因1和P-糖蛋白的表达而降低化疗药物的流出。因此,这种纳米药物可以有效地打破低氧诱导的化学耐药性,并在缺氧肿瘤中导致高效的癌症治疗。据我们所知,这是构建具有缺氧收获行为的基于AMOF的纳米药物的首次尝试。这项概念验证研究为构建低氧反应性AMOF提供了一种简单策略,并且还提供了一个独特的按需给药平台,可以有效地打破低氧诱导的化学抗药性。因此,这种纳米药物可以有效地打破低氧诱导的化学耐药性,并在缺氧肿瘤中导致高效的癌症治疗。据我们所知,这是构建具有缺氧收获行为的基于AMOF的纳米药物的首次尝试。这项概念验证研究为构建低氧反应性AMOF提供了一种简单策略,并且还提供了一个独特的按需给药平台,可以有效打破低氧诱导的化学耐药性。因此,这种纳米药物可以有效地打破低氧诱导的化学耐药性,并在缺氧肿瘤中导致高效的癌症治疗。据我们所知,这是构建具有缺氧收获行为的基于AMOF的纳米药物的首次尝试。这项概念验证研究为构建低氧反应性AMOF提供了一种简单策略,并且还提供了一个独特的按需给药平台,可以有效打破低氧诱导的化学耐药性。































 京公网安备 11010802027423号
京公网安备 11010802027423号