当前位置:
X-MOL 学术
›
Chem. Eur. J.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Competition Between Intra- and Intermolecular Hydrogen Bonding: o-Anisic Acid⋅⋅⋅Formic Acid Heterodimer.
Chemistry - A European Journal ( IF 3.9 ) Pub Date : 2019-08-01 , DOI: 10.1002/chem.201902086 Alberto Macario 1 , Susana Blanco 1 , Javix Thomas 2 , Yunjie Xu 2 , Juan Carlos López 1
Chemistry - A European Journal ( IF 3.9 ) Pub Date : 2019-08-01 , DOI: 10.1002/chem.201902086 Alberto Macario 1 , Susana Blanco 1 , Javix Thomas 2 , Yunjie Xu 2 , Juan Carlos López 1
Affiliation
|
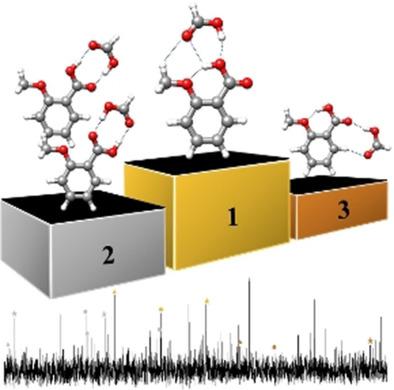
Four conformers of the heterodimer o-anisic acid-formic acid, formed in a supersonic expansion, have been probed by Fourier transform microwave spectroscopy. Two of these forms have the typical double intermolecular hydrogen-bond cyclic structure. The other two show the o-anisic acid moiety bearing a trans-COOH arrangement supported by an intramolecular O-H⋅⋅⋅O bond to the neighbor methoxy group. In these conformers, formic acid interacts with o-anisic acid mainly through an intermolecular O-H⋅⋅⋅O hydrogen bond either to the O-H or to the C=O moieties, reinforced by other weak interactions. Surprisingly, the most abundant conformer in the supersonic expansion is the complex in which the o-anisic acid is in trans arrangement with the formic acid interacting with the O-H group. Such a trans-COOH arrangement in which the intramolecular hydrogen bond dominates over the usually observed double intermolecular hydrogen bond interaction has never been observed previously in an acid-acid dimer.
中文翻译:

分子内和分子间氢键之间的竞争:邻茴香酸···甲酸异二聚体。
已经通过傅立叶变换微波光谱法探测了在超音速膨胀中形成的异二聚体邻茴香酸-甲酸的四个构象异构体。这些形式中的两种具有典型的双分子间氢键环状结构。另外两个表明邻-茴香酸部分带有一个反式-COOH排列,该排列由分子内的OH·⋅·O键连接到相邻的甲氧基上。在这些构象异构体中,甲酸主要通过与OH或与C = O部分的分子间OH·⋅·O氢键与邻茴香酸相互作用,并通过其他弱相互作用得到增强。令人惊讶地,在超音速膨胀中最丰富的构象异构体是其中邻茴香酸与甲酸与OH基相互作用的反式排列的络合物。
更新日期:2019-08-01
中文翻译:

分子内和分子间氢键之间的竞争:邻茴香酸···甲酸异二聚体。
已经通过傅立叶变换微波光谱法探测了在超音速膨胀中形成的异二聚体邻茴香酸-甲酸的四个构象异构体。这些形式中的两种具有典型的双分子间氢键环状结构。另外两个表明邻-茴香酸部分带有一个反式-COOH排列,该排列由分子内的OH·⋅·O键连接到相邻的甲氧基上。在这些构象异构体中,甲酸主要通过与OH或与C = O部分的分子间OH·⋅·O氢键与邻茴香酸相互作用,并通过其他弱相互作用得到增强。令人惊讶地,在超音速膨胀中最丰富的构象异构体是其中邻茴香酸与甲酸与OH基相互作用的反式排列的络合物。













































 京公网安备 11010802027423号
京公网安备 11010802027423号