Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Structure and autoregulation of a P4-ATPase lipid flippase
Nature ( IF 50.5 ) Pub Date : 2019-06-26 , DOI: 10.1038/s41586-019-1344-7 Milena Timcenko , Joseph A. Lyons , Dovile Januliene , Jakob J. Ulstrup , Thibaud Dieudonné , Cédric Montigny , Miriam-Rose Ash , Jesper Lykkegaard Karlsen , Thomas Boesen , Werner Kühlbrandt , Guillaume Lenoir , Arne Moeller , Poul Nissen
Nature ( IF 50.5 ) Pub Date : 2019-06-26 , DOI: 10.1038/s41586-019-1344-7 Milena Timcenko , Joseph A. Lyons , Dovile Januliene , Jakob J. Ulstrup , Thibaud Dieudonné , Cédric Montigny , Miriam-Rose Ash , Jesper Lykkegaard Karlsen , Thomas Boesen , Werner Kühlbrandt , Guillaume Lenoir , Arne Moeller , Poul Nissen
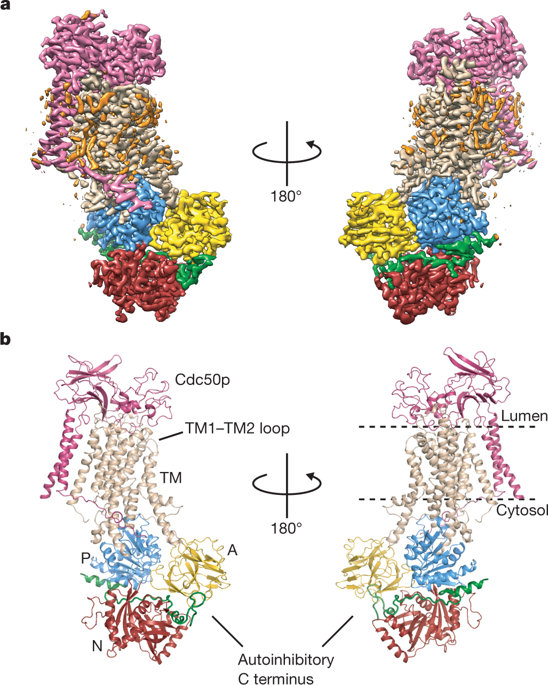
|
Type 4 P-type ATPases (P4-ATPases) are lipid flippases that drive the active transport of phospholipids from exoplasmic or luminal leaflets to cytosolic leaflets of eukaryotic membranes. The molecular architecture of P4-ATPases and the mechanism through which they recognize and transport lipids have remained unknown. Here we describe the cryo-electron microscopy structure of the P4-ATPase Drs2p–Cdc50p, a Saccharomyces cerevisiae lipid flippase that is specific to phosphatidylserine and phosphatidylethanolamine. Drs2p–Cdc50p is autoinhibited by the C-terminal tail of Drs2p, and activated by the lipid phosphatidylinositol-4-phosphate (PtdIns4P or PI4P). We present three structures that represent the complex in an autoinhibited, an intermediate and a fully activated state. The analysis highlights specific features of P4-ATPases and reveals sites of autoinhibition and PI4P-dependent activation. We also observe a putative lipid translocation pathway in this flippase that involves a conserved PISL motif in transmembrane segment 4 and polar residues of transmembrane segments 2 and 5, in particular Lys1018, in the centre of the lipid bilayer.Cryo-EM structures of the yeast P4-ATPase Drs2p–Cdc50p in three different states of activation provide insights into the function of this lipid flippase, including mechanisms of autoinhibition and PI4P-dependent activation.
中文翻译:

P4-ATPase 脂质翻转酶的结构和自动调节
4 型 P 型 ATP 酶 (P4-ATPases) 是脂质翻转酶,可驱动磷脂从外质或腔内小叶主动转运至真核细胞膜的胞质小叶。P4-ATPases 的分子结构以及它们识别和运输脂质的机制仍然未知。在这里,我们描述了 P4-ATPase Drs2p–Cdc50p 的冷冻电子显微镜结构,这是一种对磷脂酰丝氨酸和磷脂酰乙醇胺具有特异性的酿酒酵母脂质翻转酶。Drs2p–Cdc50p 被 Drs2p 的 C 端尾部自动抑制,并被脂质磷脂酰肌醇 4-磷酸(PtdIns4P 或 PI4P)激活。我们提出了三种结构,代表处于自动抑制、中间和完全激活状态的复合物。该分析突出了 P4-ATPases 的特定特征,并揭示了自身抑制和 PI4P 依赖性激活的位点。我们还观察到该翻转酶中的假定脂质易位途径,该途径涉及跨膜片段 4 中的保守 PISL 基序和跨膜片段 2 和 5 的极性残基,特别是脂双层中心的 Lys1018。 酵母的冷冻电镜结构处于三种不同激活状态的 P4-ATPase Drs2p–Cdc50p 提供了对该脂质翻转酶功能的深入了解,包括自动抑制和 PI4P 依赖性激活的机制。
更新日期:2019-06-26
中文翻译:

P4-ATPase 脂质翻转酶的结构和自动调节
4 型 P 型 ATP 酶 (P4-ATPases) 是脂质翻转酶,可驱动磷脂从外质或腔内小叶主动转运至真核细胞膜的胞质小叶。P4-ATPases 的分子结构以及它们识别和运输脂质的机制仍然未知。在这里,我们描述了 P4-ATPase Drs2p–Cdc50p 的冷冻电子显微镜结构,这是一种对磷脂酰丝氨酸和磷脂酰乙醇胺具有特异性的酿酒酵母脂质翻转酶。Drs2p–Cdc50p 被 Drs2p 的 C 端尾部自动抑制,并被脂质磷脂酰肌醇 4-磷酸(PtdIns4P 或 PI4P)激活。我们提出了三种结构,代表处于自动抑制、中间和完全激活状态的复合物。该分析突出了 P4-ATPases 的特定特征,并揭示了自身抑制和 PI4P 依赖性激活的位点。我们还观察到该翻转酶中的假定脂质易位途径,该途径涉及跨膜片段 4 中的保守 PISL 基序和跨膜片段 2 和 5 的极性残基,特别是脂双层中心的 Lys1018。 酵母的冷冻电镜结构处于三种不同激活状态的 P4-ATPase Drs2p–Cdc50p 提供了对该脂质翻转酶功能的深入了解,包括自动抑制和 PI4P 依赖性激活的机制。































 京公网安备 11010802027423号
京公网安备 11010802027423号