当前位置:
X-MOL 学术
›
Bioorgan. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Synthesis and anticancer activity of bis-benzo[d][1,3]dioxol-5-yl thiourea derivatives with molecular docking study.
Bioorganic Chemistry ( IF 4.5 ) Pub Date : 2019-06-26 , DOI: 10.1016/j.bioorg.2019.103088 Reem A K Al-Harbi 1 , Marwa A M Sh El-Sharief 2 , Samir Y Abbas 3
Bioorganic Chemistry ( IF 4.5 ) Pub Date : 2019-06-26 , DOI: 10.1016/j.bioorg.2019.103088 Reem A K Al-Harbi 1 , Marwa A M Sh El-Sharief 2 , Samir Y Abbas 3
Affiliation
|
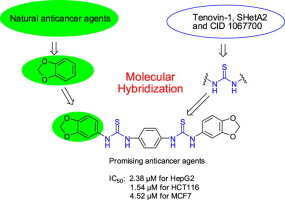
New thiourea derivatives incorporating two benzo[d][1,3]dioxol-5-yl moieties have been synthesized through the reaction of two molecules of benzo[d][1,3]dioxol-5-yl isothiocyanate with one molecule of various diamino derivatives. The synthesized compounds were examined for their cytotoxic effects using SRB assay on three cancer cell lines HepG2, HCT116 and MCF-7. Most of compounds showed significant antitumor activity and some compounds showed strong results greater than the reference drug. As example, IC50 values of 1,1'-(1,4-phenylene)bis(3-(benzo[d][1,3]dioxol-5-yl)thiourea) 5 were 2.38 µM for HepG2, 1.54 µM for HCT116 and 4.52 µM for MCF7, while the IC50 values of standard drug doxorubicin were 7.46, 8.29 and 4.56 µM, respectively. Interestingly, these compounds were non cytotoxic toward the tested normal cell line (IC50 value > 150 µM). The anticancer mechanisms were studied via EGFR inhibition assessment, annexin V-FITC apoptosis assessment, cell cycle analysis and study the effect on mitochondrial apoptosis pathway proteins Bax and Bcl-2 as well as molecular docking studies.
中文翻译:

双苯并[d] [1,3]二氧杂-5-基硫脲衍生物的合成及抗癌活性的分子对接研究。
通过两个分子的异硫氰酸苯并[d] [1,3]二氧杂-5-基的分子与一个不同分子的反应,合成了结合了两个苯并[d] [1,3]二氧杂-5-基的硫脲衍生物。二氨基衍生物。使用SRB测定法检查合成的化合物对三种癌细胞HepG2,HCT116和MCF-7的细胞毒性作用。大多数化合物显示出显着的抗肿瘤活性,某些化合物显示出比参考药物强的结果。例如,对于HepG2,1,1'-(1,4-亚苯基)双(3-(苯并[d] [1,3]二恶酚-5-基)硫脲)5的IC50值为2.38 µM,对于HepG2为1.54 µM。 MCF7的HCT116和4.52 µM,而标准药物阿霉素的IC50值分别为7.46、8.29和4.56 µM。有趣的是,这些化合物对测试的正常细胞系无细胞毒性(IC50值> 150 µM)。
更新日期:2019-06-26
中文翻译:

双苯并[d] [1,3]二氧杂-5-基硫脲衍生物的合成及抗癌活性的分子对接研究。
通过两个分子的异硫氰酸苯并[d] [1,3]二氧杂-5-基的分子与一个不同分子的反应,合成了结合了两个苯并[d] [1,3]二氧杂-5-基的硫脲衍生物。二氨基衍生物。使用SRB测定法检查合成的化合物对三种癌细胞HepG2,HCT116和MCF-7的细胞毒性作用。大多数化合物显示出显着的抗肿瘤活性,某些化合物显示出比参考药物强的结果。例如,对于HepG2,1,1'-(1,4-亚苯基)双(3-(苯并[d] [1,3]二恶酚-5-基)硫脲)5的IC50值为2.38 µM,对于HepG2为1.54 µM。 MCF7的HCT116和4.52 µM,而标准药物阿霉素的IC50值分别为7.46、8.29和4.56 µM。有趣的是,这些化合物对测试的正常细胞系无细胞毒性(IC50值> 150 µM)。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号