当前位置:
X-MOL 学术
›
ChemistrySelect
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Rational Design of Sterically Hindered and Unsymmetrical NpyNimOph Pincer‐Type Ligands and Their Palladium(II) Complexes: Catalytic Applications in Suzuki–Miyaura Reaction and Allylation of Aldehydes
ChemistrySelect ( IF 1.9 ) Pub Date : 2019-06-25 , DOI: 10.1002/slct.201900946
Ankur Maji 1 , Ovender Singh 1 , Sweety Rathi 1 , U. P. Singh 1 , Kaushik Ghosh 1
ChemistrySelect ( IF 1.9 ) Pub Date : 2019-06-25 , DOI: 10.1002/slct.201900946
Ankur Maji 1 , Ovender Singh 1 , Sweety Rathi 1 , U. P. Singh 1 , Kaushik Ghosh 1
Affiliation
|
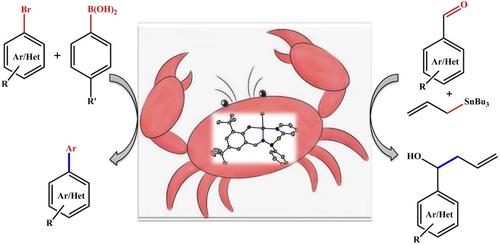
Palladium(II) complexes 1–4 [Pd(L1 to L4)Cl] derived from pincer‐type tridentate ligands having NpyNimOph donors were synthesized from ligands L1H to L4H (where L1H=(2‐phenyl‐2‐(pyridin‐2‐ylmethyl)hydrazono)methyl)phenol, L2H =[2‐((2‐phenyl‐2‐(pyridinyl‐2‐ylmethyl) hydrazono) methyl) phenol], L3H=[2‐((2‐benzyl‐2‐phenylhydrazono) methyl)‐4,6‐di‐tert‐butylphenol], and L4H=[2‐((2‐benzyl‐2‐phenylhydrazono)methyl)‐6‐(tert‐butyl)‐4‐methoxy phenol where H stands for dissociable proton)in quantitative yields. Synthesized ligands L1H to L4H and corresponding palladium(II) complexes 1–4 were characterized by NMR, IR and HR‐MS spectroscopic studies. Molecular structures of ligand L4H and complexes 1 and 3 were determined by X‐ray crystallography. Complexes 1–4 were utilized as catalysts to investigate Suzuki–Miyaura cross‐coupling reaction upon catalyst loading 0.01 mol%. These catalysts also catalysed allylation of aldehydes upto 95% yield upon catalyst loading 0.5 mol% with broad substrate scopes. Theoretical and experimental investigation was performed to speculate reaction pathway of Suzuki–Miyaura cross‐coupling reaction. A reaction model has also proposed for allylation of aldehydes the presence of allyltributylstannane.
中文翻译:

立体受阻和非对称NpyNimOph钳型配体及其钯(II)配合物的合理设计:铃木-宫浦反应和醛烯丙基化的催化应用
从具有N py N im O ph供体的钳型三齿配体衍生的钯(II)配合物1-4 [Pd(L 1至L 4)Cl]由配体L 1 H至L 4 H合成(其中L 1 H =(2-苯基-2-(吡啶-2-基甲基)(恶唑啉基)甲基)苯酚,L 2 H = [2-((2-苯基-2-(吡啶基-2-基甲基)肼基)甲基)苯酚], L 3 H = [2-(((2-苄基-2-苯基肼基)甲基)-4,6-二叔丁基苯酚],L 4 H = [2-(((2-苄基-2-苯基肼基)甲基] )-6-(叔丁基)-4-甲氧基苯酚,其中H表示可解离的质子),定量收率。合成配体L1 H至L 4 H和相应的钯(II)配合物1-4通过NMR,IR和HR-MS光谱研究表征。X射线晶体学测定了配体L 4 H以及配合物1和3的分子结构。配合物1-4用作催化剂以研究铃木-宫浦在催化剂负载量为0.01 mol%时的交叉偶联反应。这些催化剂在催化剂负载量为0.5 mol%,底物范围广的情况下,还催化醛的烯丙基化反应,收率高达95%。进行了理论和实验研究,推测了铃木-宫浦交叉偶联反应的反应路径。还提出了在烯丙基三丁基锡烷的存在下使醛烯丙基化的反应模型。
更新日期:2019-06-25
中文翻译:

立体受阻和非对称NpyNimOph钳型配体及其钯(II)配合物的合理设计:铃木-宫浦反应和醛烯丙基化的催化应用
从具有N py N im O ph供体的钳型三齿配体衍生的钯(II)配合物1-4 [Pd(L 1至L 4)Cl]由配体L 1 H至L 4 H合成(其中L 1 H =(2-苯基-2-(吡啶-2-基甲基)(恶唑啉基)甲基)苯酚,L 2 H = [2-((2-苯基-2-(吡啶基-2-基甲基)肼基)甲基)苯酚], L 3 H = [2-(((2-苄基-2-苯基肼基)甲基)-4,6-二叔丁基苯酚],L 4 H = [2-(((2-苄基-2-苯基肼基)甲基] )-6-(叔丁基)-4-甲氧基苯酚,其中H表示可解离的质子),定量收率。合成配体L1 H至L 4 H和相应的钯(II)配合物1-4通过NMR,IR和HR-MS光谱研究表征。X射线晶体学测定了配体L 4 H以及配合物1和3的分子结构。配合物1-4用作催化剂以研究铃木-宫浦在催化剂负载量为0.01 mol%时的交叉偶联反应。这些催化剂在催化剂负载量为0.5 mol%,底物范围广的情况下,还催化醛的烯丙基化反应,收率高达95%。进行了理论和实验研究,推测了铃木-宫浦交叉偶联反应的反应路径。还提出了在烯丙基三丁基锡烷的存在下使醛烯丙基化的反应模型。

































 京公网安备 11010802027423号
京公网安备 11010802027423号