当前位置:
X-MOL 学术
›
ChemCatChem
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Insight into the Role of Water on the Methylation of Hexamethylbenzene in H‐SAPO‐34 from First Principle Molecular Dynamics Simulations
ChemCatChem ( IF 3.8 ) Pub Date : 2019-07-09 , DOI: 10.1002/cctc.201900618 Simon Bailleul 1 , Sven M. J. Rogge 1 , Louis Vanduyfhuys 1 , Veronique Van Speybroeck 1
ChemCatChem ( IF 3.8 ) Pub Date : 2019-07-09 , DOI: 10.1002/cctc.201900618 Simon Bailleul 1 , Sven M. J. Rogge 1 , Louis Vanduyfhuys 1 , Veronique Van Speybroeck 1
Affiliation
|
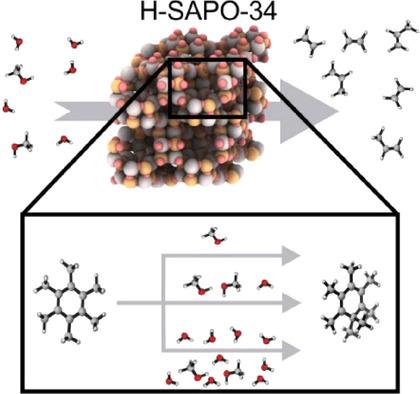
The methylation of hexamethylbenzene with methanol is one of the key reactions in the methanol‐to‐olefins hydrocarbon pool reaction cycle taking place over the industrially relevant H‐SAPO‐34 zeolite. This methylation reaction can occur either via a concerted or via a stepwise mechanism, the latter being the preferred pathway at higher temperatures. Herein, we systematically investigate how a complex reaction environment with additional water molecules and higher concentrations of Brønsted acid sites in the zeolite impacts the reaction mechanism. To this end, first principle molecular dynamics simulations are performed using enhanced sampling methods to characterize the reactants and products in the catalyst pores and to construct the free energy profiles. The most prominent effect of the dynamic sampling of the reaction path is the stabilization of the product region where water is formed, which can either move freely in the pores of the zeolite or be stabilized through hydrogen bonding with the other protic molecules. These protic molecules also stabilize the deprotonated Brønsted acid site, created due to the formation of the heptamethylbenzenium cation, via a Grotthuss‐type mechanism. Our results provide fundamental insight in the experimental parameters that impact the methylation of hexamethylbenzene in H‐SAPO‐34, especially highlighting and rationalizing the crucial role of water in one of the main reactions of the aromatics‐based reaction cycle.
中文翻译:

通过第一原理分子动力学模拟洞察水对H-SAPO-34中六甲基苯甲基化的作用
六甲基苯与甲醇的甲基化是发生在与工业相关的H-SAPO-34沸石上的甲醇制烯烃烃池反应循环中的关键反应之一。该甲基化反应可以通过协同作用或通过逐步的机制发生,后者是较高温度下的优选途径。在本文中,我们系统地研究了在沸石中具有更多水分子和更高浓度的布朗斯台德酸位点的复杂反应环境如何影响反应机理。为此,使用增强的采样方法进行第一原理分子动力学模拟,以表征催化剂孔中的反应物和产物并构建自由能分布。动态采样反应路径的最显着效果是稳定了形成水的产物区域,该产物可以在沸石的孔中自由移动,也可以通过与其他质子分子的氢键键合而稳定。这些质子分子还通过格罗素斯型机制稳定了去质子化的布朗斯台德酸位点,该位点是由于七甲基苯甲铵阳离子的形成而产生的。我们的结果为影响H-SAPO-34中六甲基苯甲基化的实验参数提供了基础见解,尤其是突出并合理化了水在基于芳烃的反应循环的主要反应之一中的关键作用。它可以在沸石的孔中自由移动,也可以通过与其他质子分子的氢键键合而稳定。这些质子分子还通过格罗素斯型机制稳定了去质子化的布朗斯台德酸位点,该位点是由于七甲基苯甲铵阳离子的形成而产生的。我们的结果为影响H-SAPO-34中六甲基苯甲基化的实验参数提供了基础见解,尤其是突出并合理化了水在基于芳烃的反应循环的主要反应之一中的关键作用。它可以在沸石的孔中自由移动,也可以通过与其他质子分子的氢键键合而稳定。这些质子分子还通过格罗素斯型机制稳定了去质子化的布朗斯台德酸位点,该位点是由于七甲基苯甲铵阳离子的形成而产生的。我们的结果为影响H-SAPO-34中六甲基苯甲基化的实验参数提供了基础见解,尤其是突出并合理化了水在基于芳烃的反应循环的主要反应之一中的关键作用。
更新日期:2019-07-09
中文翻译:

通过第一原理分子动力学模拟洞察水对H-SAPO-34中六甲基苯甲基化的作用
六甲基苯与甲醇的甲基化是发生在与工业相关的H-SAPO-34沸石上的甲醇制烯烃烃池反应循环中的关键反应之一。该甲基化反应可以通过协同作用或通过逐步的机制发生,后者是较高温度下的优选途径。在本文中,我们系统地研究了在沸石中具有更多水分子和更高浓度的布朗斯台德酸位点的复杂反应环境如何影响反应机理。为此,使用增强的采样方法进行第一原理分子动力学模拟,以表征催化剂孔中的反应物和产物并构建自由能分布。动态采样反应路径的最显着效果是稳定了形成水的产物区域,该产物可以在沸石的孔中自由移动,也可以通过与其他质子分子的氢键键合而稳定。这些质子分子还通过格罗素斯型机制稳定了去质子化的布朗斯台德酸位点,该位点是由于七甲基苯甲铵阳离子的形成而产生的。我们的结果为影响H-SAPO-34中六甲基苯甲基化的实验参数提供了基础见解,尤其是突出并合理化了水在基于芳烃的反应循环的主要反应之一中的关键作用。它可以在沸石的孔中自由移动,也可以通过与其他质子分子的氢键键合而稳定。这些质子分子还通过格罗素斯型机制稳定了去质子化的布朗斯台德酸位点,该位点是由于七甲基苯甲铵阳离子的形成而产生的。我们的结果为影响H-SAPO-34中六甲基苯甲基化的实验参数提供了基础见解,尤其是突出并合理化了水在基于芳烃的反应循环的主要反应之一中的关键作用。它可以在沸石的孔中自由移动,也可以通过与其他质子分子的氢键键合而稳定。这些质子分子还通过格罗素斯型机制稳定了去质子化的布朗斯台德酸位点,该位点是由于七甲基苯甲铵阳离子的形成而产生的。我们的结果为影响H-SAPO-34中六甲基苯甲基化的实验参数提供了基础见解,尤其是突出并合理化了水在基于芳烃的反应循环的主要反应之一中的关键作用。

































 京公网安备 11010802027423号
京公网安备 11010802027423号