European Journal of Medicinal Chemistry ( IF 6.0 ) Pub Date : 2019-06-25 , DOI: 10.1016/j.ejmech.2019.06.056 Eufrânio N. da Silva Júnior , Guilherme A.M. Jardim , Claus Jacob , Uttam Dhawa , Lutz Ackermann , Solange L. de Castro
|
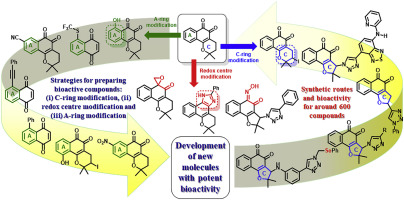
Naphthoquinones are of key importance in organic synthesis and medicinal chemistry. In the last few years, various synthetic routes have been developed to prepare bioactive compounds derived or based on lapachones. In this sense, this review is mainly focused on the synthetic aspects and strategies used for the design of these compounds on the basis of their biological activities for the development of drugs against the neglected diseases leishmaniases and Chagas disease and also cancer. Three strategies used to develop bioactive quinones are discussed and categorized: (i) C-ring modification, (ii) redox centre modification and (iii) A-ring modification. Framed within these strategies for the development of naphthoquinoidal compounds against T. cruzi. Leishmania and cancer, reactions including copper-catalyzed azide-alkyne cycloaddition (click chemistry), palladium-catalysed cross couplings, C–H activation reactions, Ullmann couplings and heterocyclisations reported up to July 2019 will be discussed. The aim of derivatisation is the generation of novel molecules that can potentially inhibit cellular organelles/processes, generate reactive oxygen species and increase lipophilicity to enhance penetration through the plasma membrane. Modified lapachones have emerged as promising prototypes for the development of drugs against leishmaniases, Chagas disease and cancer.
中文翻译:

醌的合成具有突出的生物学应用:对生物活性化合物策略的重要更新,重点是拉帕酮
萘醌在有机合成和药物化学中至关重要。在最近几年中,已开发出各种合成途径来制备衍生自或基于拉帕酮的生物活性化合物。从这个意义上讲,本综述主要基于合成这些化合物的生物学方面和策略,这些化合物的设计基于其生物活性,用于开发针对被忽视的疾病利什曼病和恰加斯病以及癌症的药物。对开发生物活性醌的三种策略进行了讨论和分类:(i)C环修饰,(ii)氧化还原中心修饰和(iii)A环修饰。在这些战略的框架内发展对抗克鲁氏酵母的萘醌类化合物。利什曼原虫与癌症相关的反应将进行讨论,包括截至2019年7月的铜催化的叠氮化物-炔烃环加成反应(点击化学反应),钯催化的交叉偶联,CH活化反应,Ullmann偶联和杂环化反应。衍生化的目的是产生可以潜在地抑制细胞器/过程,产生活性氧物种并增加亲脂性以增强通过质膜的渗透力的新型分子。修饰的拉帕酮已成为开发抗利什曼病,查加斯病和癌症药物的有希望的原型。




















































 京公网安备 11010802027423号
京公网安备 11010802027423号