当前位置:
X-MOL 学术
›
Cell Death Dis.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Mitochondrial miR-762 regulates apoptosis and myocardial infarction by impairing ND2.
Cell Death & Disease ( IF 8.1 ) Pub Date : 2019-06-24 , DOI: 10.1038/s41419-019-1734-7 Kaowen Yan 1 , Tao An 2 , Mei Zhai 2 , Yan Huang 2 , Qi Wang 1 , Yunhong Wang 2 , Rongcheng Zhang 2 , Tao Wang 1 , Jing Liu 1 , Yuhui Zhang 2 , Jian Zhang 2 , Kun Wang 1
Cell Death & Disease ( IF 8.1 ) Pub Date : 2019-06-24 , DOI: 10.1038/s41419-019-1734-7 Kaowen Yan 1 , Tao An 2 , Mei Zhai 2 , Yan Huang 2 , Qi Wang 1 , Yunhong Wang 2 , Rongcheng Zhang 2 , Tao Wang 1 , Jing Liu 1 , Yuhui Zhang 2 , Jian Zhang 2 , Kun Wang 1
Affiliation
|
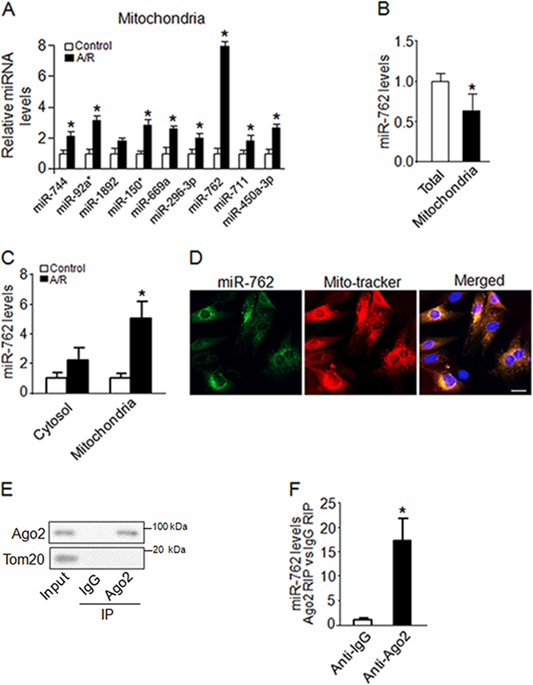
Mitochondrial dysfunction plays a major role in the pathogenesis of cardiovascular diseases. MicroRNAs (miRNAs) are small RNAs that act as negative regulators of gene expression, but how miRNAs affect mitochondrial function in the heart is unclear. Using a miRNA microarray assay, we found that miR-762 predominantly translocated in the mitochondria and was significantly upregulated upon anoxia/reoxygenation (A/R) treatment. Knockdown of endogenous miR-762 significantly attenuated the decrease in intracellular ATP levels, the increase in ROS levels, the decrease in mitochondrial complex I enzyme activity and the increase in apoptotic cell death in cardiomyocytes, which was induced by A/R treatment. In addition, knockdown of miR-762 ameliorated myocardial ischemia/reperfusion (I/R) injury in mice. Mechanistically, we showed that enforced expression of miR-762 dramatically decreased the protein levels of endogenous NADH dehydrogenase subunit 2 (ND2) but had no effect on the transcript levels of ND2. The luciferase reporter assay showed that miR-762 bound to the coding sequence of ND2. In addition, knockdown of endogenous ND2 significantly decreased intracellular ATP levels, increased ROS levels, reduced mitochondrial complex I enzyme activity and increased apoptotic cell death in cardiomyocytes, which was induced by A/R treatment. Furthermore, we found that the inhibitory effect of miR-762 downregulation was attenuated by ND2 knockdown. Thus, our findings suggest that miR-762 participates in the regulation of mitochondrial function and cardiomyocyte apoptosis by ND2, a core assembly subunit of mitochondrial complex I. Our results revealed that mitochondrial miR-762, as a new player in mitochondrial dysfunction, may provide a new therapeutic target for myocardial infarction.
中文翻译:

线粒体miR-762通过损害ND2来调节细胞凋亡和心肌梗塞。
线粒体功能障碍在心血管疾病的发病机理中起主要作用。微小RNA(miRNA)是充当基因表达负调控因子的小RNA,但尚不清楚miRNA如何影响心脏线粒体功能。使用miRNA芯片检测,我们发现miR-762主要位于线粒体中,并且在缺氧/复氧(A / R)处理后显着上调。内源性miR-762的抑制显着减弱了A / R处理诱导的心肌细胞内ATP水平的下降,ROS水平的上升,线粒体复合物I酶活性的下降以及心肌细胞凋亡的增加。另外,敲低miR-762可以减轻小鼠的心肌缺血/再灌注(I / R)损伤。机械上,我们表明,miR-762的强制表达显着降低了内源性NADH脱氢酶亚基2(ND2)的蛋白水平,但对ND2的转录水平没有影响。荧光素酶报告基因测定表明,miR-762与ND2的编码序列结合。另外,内源性ND2的敲低显着降低了细胞内ATP水平,增加了ROS水平,降低了线粒体复合物I酶的活性,并增加了A / R处理诱导的心肌细胞凋亡的细胞死亡。此外,我们发现,ND2敲低减弱了miR-762下调的抑制作用。因此,我们的发现表明,miR-762通过线粒体复合体I的核心装配亚基ND2参与线粒体功能和心肌细胞凋亡的调节。
更新日期:2019-06-24
中文翻译:

线粒体miR-762通过损害ND2来调节细胞凋亡和心肌梗塞。
线粒体功能障碍在心血管疾病的发病机理中起主要作用。微小RNA(miRNA)是充当基因表达负调控因子的小RNA,但尚不清楚miRNA如何影响心脏线粒体功能。使用miRNA芯片检测,我们发现miR-762主要位于线粒体中,并且在缺氧/复氧(A / R)处理后显着上调。内源性miR-762的抑制显着减弱了A / R处理诱导的心肌细胞内ATP水平的下降,ROS水平的上升,线粒体复合物I酶活性的下降以及心肌细胞凋亡的增加。另外,敲低miR-762可以减轻小鼠的心肌缺血/再灌注(I / R)损伤。机械上,我们表明,miR-762的强制表达显着降低了内源性NADH脱氢酶亚基2(ND2)的蛋白水平,但对ND2的转录水平没有影响。荧光素酶报告基因测定表明,miR-762与ND2的编码序列结合。另外,内源性ND2的敲低显着降低了细胞内ATP水平,增加了ROS水平,降低了线粒体复合物I酶的活性,并增加了A / R处理诱导的心肌细胞凋亡的细胞死亡。此外,我们发现,ND2敲低减弱了miR-762下调的抑制作用。因此,我们的发现表明,miR-762通过线粒体复合体I的核心装配亚基ND2参与线粒体功能和心肌细胞凋亡的调节。































 京公网安备 11010802027423号
京公网安备 11010802027423号