Journal of Molecular Biology ( IF 4.7 ) Pub Date : 2019-06-24 , DOI: 10.1016/j.jmb.2019.06.012 Lina Herhaus 1 , Henry van den Bedem 2 , Sean Tang 3 , Innokentiy Maslennikov 3 , Soichi Wakatsuki 4 , Ivan Dikic 5 , Simin Rahighi 3
|
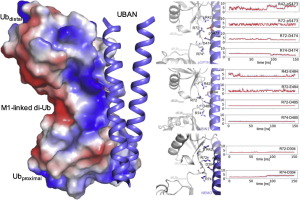
Although the Ub-binding domain in ABIN proteins and NEMO (UBAN) is highly conserved, UBAN-containing proteins exhibit different Ub-binding properties, resulting in their diverse biological roles. Post-translational modifications further control UBAN domain specificity for poly-Ub chains. However, precisely, how the UBAN domain structurally confers such functional diversity remains poorly understood. Here we report crystal structures of ABIN-1 alone and in complex with one or two M1-linked di-Ub chains. ABIN-1 UBAN forms a homo-dimer that provides two symmetrical Ub-binding sites on either side of the coiled-coil structure. Moreover, crystal structures of ABIN1 UBAN in complex with di-Ub chains reveal a concentration-dependency of UBAN/di-Ub binding stoichiometry. Analysis of UBAN/M1-linked di-Ub binding characteristics indicates that phosphorylated S473 in OPTN and its corresponding phospho-mimetic residue in ABIN-1 (E484) are essential for high affinity interactions with M1-linked Ub chains. Also, a phospho-mimetic mutation of A303 in NEMO, corresponding to S473 of OPTN, increases binding affinity for M1-linked Ub chains. These findings are in line with the diverse physiological roles of UBAN domains, as phosphorylation of OPTN UBAN is required to enhance its binding to Ub during mitophagy.
中文翻译:

天然和磷酸化的UBAN域对M1连接的泛素链的分子识别。
尽管ABIN蛋白和NEMO(UBAN)中的Ub结合结构域是高度保守的,但含UBAN的蛋白却表现出不同的Ub结合特性,从而导致其多种生物学作用。翻译后修饰进一步控制了多Ub链的UBAN域特异性。但是,确切地讲,UBAN域如何在结构上赋予这种功能多样性仍然知之甚少。在这里,我们报告了单独的ABIN-1晶体结构以及与一个或两个M1连接的Di-Ub链复合的晶体结构。ABIN-1 UBAN形成同型二聚体,该同型二聚体在卷曲螺旋结构的任一侧提供两个对称的Ub结合位点。而且,与di-Ub链复合的ABIN1 UBAN的晶体结构显示了UBAN / di-Ub结合化学计量的浓度依赖性。UBAN / M1联结的Di-Ub结合特性分析表明,OPTN中的S473磷酸化以及ABIN-1(E484)中相应的磷酸化残基对与M1联结的Ub链的高亲和力相互作用至关重要。同样,NEMO中的A303磷酸化模拟突变(对应于OPTN的S473)增加了与M1连接的Ub链的结合亲和力。这些发现与UBAN域的多种生理作用一致,因为在线粒体吞噬过程中需要OPTN UBAN的磷酸化以增强其与Ub的结合。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号