当前位置:
X-MOL 学术
›
Nat. Struct. Mol. Biol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Structural basis of tubulin detyrosination by vasohibins.
Nature Structural & Molecular Biology ( IF 12.5 ) Pub Date : 2019-06-24 , DOI: 10.1038/s41594-019-0242-x
Faxiang Li 1, 2 , Yingjie Hu 1, 2 , Shutao Qi 1, 2 , Xuelian Luo 1, 3 , Hongtao Yu 1, 2
Nature Structural & Molecular Biology ( IF 12.5 ) Pub Date : 2019-06-24 , DOI: 10.1038/s41594-019-0242-x
Faxiang Li 1, 2 , Yingjie Hu 1, 2 , Shutao Qi 1, 2 , Xuelian Luo 1, 3 , Hongtao Yu 1, 2
Affiliation
|
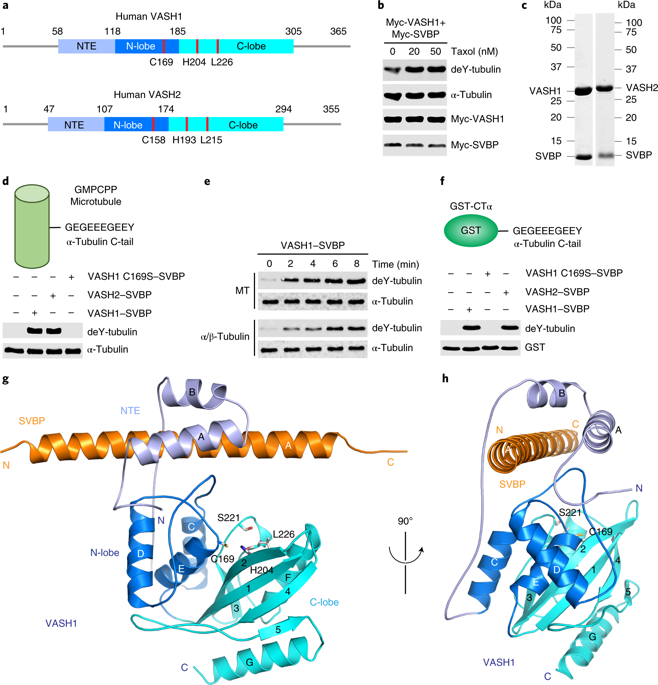
Microtubules are regulated by post-translational modifications of tubulin. The ligation and cleavage of the carboxy-terminal tyrosine of α-tubulin impact microtubule functions during mitosis, cardiomyocyte contraction and neuronal processes. Tubulin tyrosination and detyrosination are mediated by tubulin tyrosine ligase and the recently discovered tubulin detyrosinases, vasohibin 1 and 2 (VASH1 and VASH2) bound to the small vasohibin-binding protein (SVBP). Here, we report the crystal structures of human VASH1-SVBP alone, in complex with a tyrosine-derived covalent inhibitor and bound to the natural product parthenolide. The structures and subsequent mutagenesis analyses explain the requirement for SVBP during tubulin detyrosination, and reveal the basis for the recognition of the C-terminal tyrosine and the acidic α-tubulin tail by VASH1. The VASH1-SVBP-parthenolide structure provides a framework for designing more effective chemical inhibitors of vasohibins, which can be valuable for dissecting their biological functions and may have therapeutic potential.
中文翻译:

血管抑制素使微管蛋白脱酪氨酸的结构基础。
微管通过微管蛋白的翻译后修饰来调节。α-微管蛋白的羧基末端酪氨酸的连接和裂解影响有丝分裂,心肌细胞收缩和神经元过程中的微管功能。微管蛋白酪氨酸连接酶和最近发现的微管蛋白脱酪氨酸酶,与小血管抑制素结合蛋白(SVBP)结合的血管抑制素1和2(VASH1和VASH2)介导微管蛋白的酪氨酸化和脱酪氨酸作用。在这里,我们报告了人类VASH1-SVBP单独的晶体结构,与酪氨酸衍生的共价抑制剂复合,并与天然产物小白菊内酯结合。结构和随后的诱变分析解释了微管蛋白脱酪氨酸过程中对SVBP的需求,并揭示了VASH1识别C端酪氨酸和酸性α-微管蛋白尾巴的基础。
更新日期:2019-06-24
中文翻译:

血管抑制素使微管蛋白脱酪氨酸的结构基础。
微管通过微管蛋白的翻译后修饰来调节。α-微管蛋白的羧基末端酪氨酸的连接和裂解影响有丝分裂,心肌细胞收缩和神经元过程中的微管功能。微管蛋白酪氨酸连接酶和最近发现的微管蛋白脱酪氨酸酶,与小血管抑制素结合蛋白(SVBP)结合的血管抑制素1和2(VASH1和VASH2)介导微管蛋白的酪氨酸化和脱酪氨酸作用。在这里,我们报告了人类VASH1-SVBP单独的晶体结构,与酪氨酸衍生的共价抑制剂复合,并与天然产物小白菊内酯结合。结构和随后的诱变分析解释了微管蛋白脱酪氨酸过程中对SVBP的需求,并揭示了VASH1识别C端酪氨酸和酸性α-微管蛋白尾巴的基础。































 京公网安备 11010802027423号
京公网安备 11010802027423号