当前位置:
X-MOL 学术
›
Ind. Eng. Chem. Res.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
New Process for 2,6-Dimethylnaphthalene Synthesis by Using C10 Aromatics as Solvent and Transmethylation-Agentia: High-Efficiency and Peculiar Subarea-Catalysis over Shape-Selective ZSM-5/Beta Catalyst
Industrial & Engineering Chemistry Research ( IF 3.8 ) Pub Date : 2019-07-02 , DOI: 10.1021/acs.iecr.9b01596 Junhui Li 1, 2 , Qing Gong 1 , Hua Lian 1 , Zhonghua Hu 2 , Zhirong Zhu 2
Industrial & Engineering Chemistry Research ( IF 3.8 ) Pub Date : 2019-07-02 , DOI: 10.1021/acs.iecr.9b01596 Junhui Li 1, 2 , Qing Gong 1 , Hua Lian 1 , Zhonghua Hu 2 , Zhirong Zhu 2
Affiliation
|
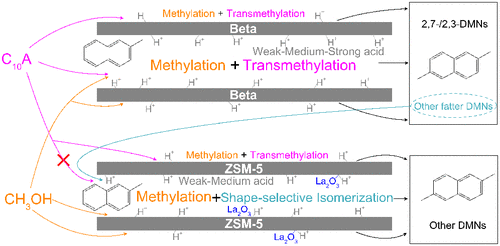
A new methylation (2-methylnaphthalene (2-MN) with methanol) process using low-value C10 aromatics (C10A) as solvent and transmethylation-agentia was developed for the lower-cost synthesis of 2,6-dimethylnaphthalene (2,6-DMN), by using a ZSM-5/Beta composite catalyst. Especially, there is a peculiar subarea-catalysis over this catalyst due to both the reactant/product shape selectivity and the ingenious acidity design. Concretely, methylation (2-MN with methanol) and transmethylation (C10A with 2-MN) are catalyzed by the weak + medium + strong acid sites inside 12-ring pore and on the external surface of zeolites, while almost no transmethylation (but methylation and second shape-selective isomerization of fatter DMNs to β,β-DMN) occurs inside the smaller 10-ring pore (rejecting larger-molecular C10A) with mainly weak–medium acidity. Here, both the high-efficiency synergy of methylation with transmethylation and the meritorious isomerization incubated a 2-MN conversion of 52.98% and a 2,6-DMN yield of 11.01%. Our work may provide a promising model for the rational design of an excellent catalyst for a complicated reaction system.
中文翻译:

以C10芳烃为溶剂和Transmethylation-Agentia合成2,6-二甲基萘的新工艺:形状选择性ZSM-5 /β催化剂上的高效和奇特的分区催化
以低成本的C 10芳族化合物(C 10 A)为溶剂和反甲基化试剂,开发了一种新的甲基化(2-甲基萘(2-MN)与甲醇)工艺,以低成本合成2,6-二甲基萘(2 (6-DMN),通过使用ZSM-5 /β复合催化剂。特别地,由于反应物/产物形状的选择性和巧妙的酸度设计,在该催化剂上存在独特的分区催化。具体而言,甲基化(2-MN加甲醇)和反甲基化(C 10带有2-MN的A)被12环孔内和沸石外表面上的弱+中+强酸部位催化,而几乎没有甲基化(而是脂肪性DMNs的甲基化和第二次形状选择性异构化为β,β -DMN)发生在较小的10环孔内(拒绝较大分子的C 10 A),主要是弱酸性。在此,甲基化与反甲基化的高效协同作用和优异的异构化反应均使5-29.8%的2-MN转化率和11.01%的2,6-DMN转化率产生了影响。我们的工作可能为合理设计复杂反应系统的优良催化剂提供有希望的模型。
更新日期:2019-07-03
中文翻译:

以C10芳烃为溶剂和Transmethylation-Agentia合成2,6-二甲基萘的新工艺:形状选择性ZSM-5 /β催化剂上的高效和奇特的分区催化
以低成本的C 10芳族化合物(C 10 A)为溶剂和反甲基化试剂,开发了一种新的甲基化(2-甲基萘(2-MN)与甲醇)工艺,以低成本合成2,6-二甲基萘(2 (6-DMN),通过使用ZSM-5 /β复合催化剂。特别地,由于反应物/产物形状的选择性和巧妙的酸度设计,在该催化剂上存在独特的分区催化。具体而言,甲基化(2-MN加甲醇)和反甲基化(C 10带有2-MN的A)被12环孔内和沸石外表面上的弱+中+强酸部位催化,而几乎没有甲基化(而是脂肪性DMNs的甲基化和第二次形状选择性异构化为β,β -DMN)发生在较小的10环孔内(拒绝较大分子的C 10 A),主要是弱酸性。在此,甲基化与反甲基化的高效协同作用和优异的异构化反应均使5-29.8%的2-MN转化率和11.01%的2,6-DMN转化率产生了影响。我们的工作可能为合理设计复杂反应系统的优良催化剂提供有希望的模型。





















































 京公网安备 11010802027423号
京公网安备 11010802027423号