Scientific Reports ( IF 3.8 ) Pub Date : 2019-06-24 , DOI: 10.1038/s41598-019-45444-0 Ian S Miller 1 , Liam P Shiels 1 , Emer Conroy 2 , Kate Connor 1 , Patrick Dicker 3 , William M Gallagher 2 , Norma O' Donovan 4 , Robert S Kerbel 5 , John Crown 4 , Annette T Byrne 1, 2
|
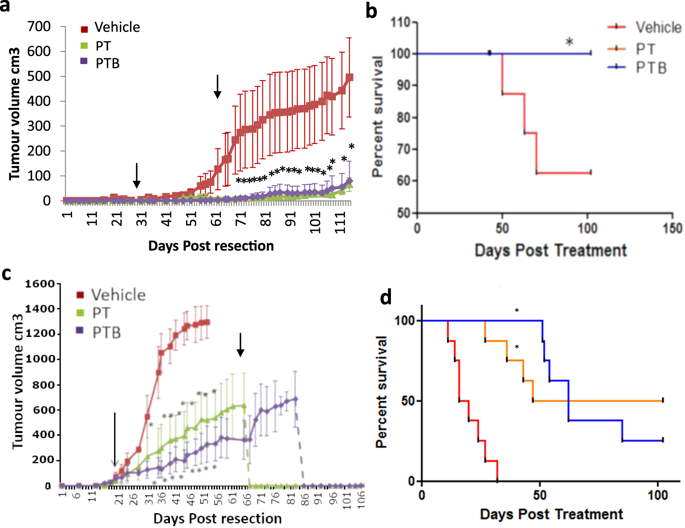
Angiogenesis is a key tumor microenvironment (TME) event underpinning tumor growth and metastasis. Nevertheless, the relatively poor performance of anti-angiogenic therapies in clinical trials compared to pre-clinical studies implies that classical subcutaneous xenograft models have limited predictive potential in this setting. To address this issue, we established orthotopic surgical resection models of breast cancer, which replicate the phenotype of clinical post-resection micro-metastasis. To demonstrate the power and precision of these models, we recapitulated the BETH adjuvant trial (NCT00625898) where the addition of bevacizumab (BVZ) to chemotherapy plus trastuzumab (Trast) failed to provide additional benefit. SCID mice were orthotopically implanted with bioluminescent Her2+ MDA-MB-231 or HCC1954 cells and tumors resected c.5 weeks later. Following resection, mice were treated with 10 mg/kg Trast +5 mg/kg paclitaxel (PAC) IP once weekly for 6 cycles +/− weekly BVZ (5 mg/kg IP). Metastasis was monitored by imaging. Using these models our data confirms that the addition of the anti-angiogenic antibody BVZ to adjuvant Trast + chemotherapy provides no additional benefit compared with Trast + chemotherapy alone. Previous studies using non-resection subcutaneously engrafted xenografts failed to predict this outcome. Our results provide compelling evidence for the utility of cell line xenograft resection models to predict clinical outcome for TME targeting agents.
中文翻译:

细胞系异种移植切除模型可询问肿瘤微环境靶向剂的耐久性。
血管生成是支撑肿瘤生长和转移的关键肿瘤微环境(TME)事件。然而,与临床前研究相比,抗血管生成疗法在临床试验中的表现相对较差,这表明经典皮下异种移植模型在这种情况下的预测潜力有限。为了解决这个问题,我们建立了乳腺癌的原位手术切除模型,该模型复制了临床切除后微转移的表型。为了证明这些模型的功能和精度,我们总结了BETH佐剂试验(NCT00625898),其中在化学疗法中加贝伐单抗(BVZ)加曲妥珠单抗(Trast)无法提供额外的益处。将SCID小鼠原位植入生物发光的Her2 +约5周后切除MDA-MB-231或HCC1954细胞和肿瘤。切除后,每周一次用10 mg / kg Trast +5 mg / kg紫杉醇(PAC)IP治疗小鼠,连续6个周期+/- BVZ(5 mg / kg IP)。通过成像监测转移。使用这些模型,我们的数据证实与单独的Trast +化疗相比,在佐剂Trast +化疗中添加抗血管生成抗体BVZ不会提供任何额外的益处。先前使用非切除皮下植入异种移植物的研究未能预测这一结果。我们的结果提供了令人信服的证据,证明细胞系异种移植切除模型可用于预测TME靶向药物的临床结果。






























 京公网安备 11010802027423号
京公网安备 11010802027423号