Nature Communications ( IF 14.7 ) Pub Date : 2019-06-24 , DOI: 10.1038/s41467-019-10696-x Yibing Deng 1 , Tao Wu 1 , Mengdi Wang 1 , Shengchao Shi 1 , Guodong Yuan 1 , Xi Li 1 , Hanchung Chong 2, 3 , Bin Wu 2, 3 , Peng Zheng 1
|
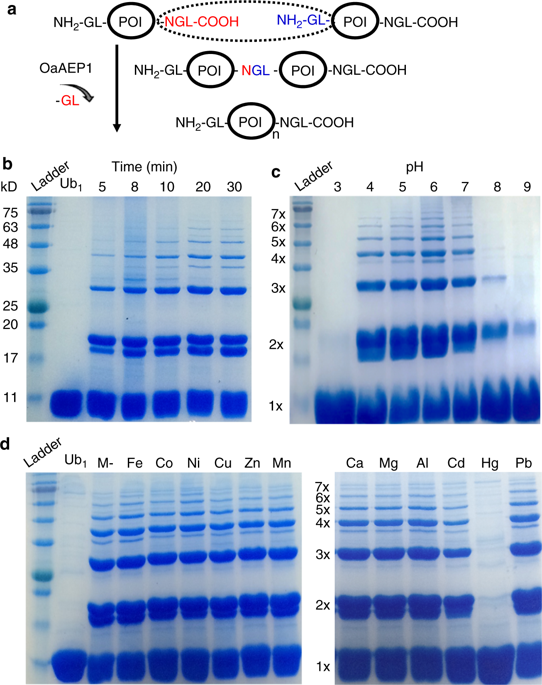
The recent development of chemical and bio-conjugation techniques allows for the engineering of various protein polymers. However, most of the polymerization process is difficult to control. To meet this challenge, we develop an enzymatic procedure to build polyprotein using the combination of a strict protein ligase OaAEP1 (Oldenlandia affinis asparaginyl endopeptidases 1) and a protease TEV (tobacco etch virus). We firstly demonstrate the use of OaAEP1-alone to build a sequence-uncontrolled ubiquitin polyprotein and covalently immobilize the coupled protein on the surface. Then, we construct a poly-metalloprotein, rubredoxin, from the purified monomer. Lastly, we show the feasibility of synthesizing protein polymers with rationally-controlled sequences by the synergy of the ligase and protease, which are verified by protein unfolding using atomic force microscopy-based single-molecule force spectroscopy (AFM-SMFS). Thus, this study provides a strategy for polyprotein engineering and immobilization.
中文翻译:

酶促生物合成和多蛋白固定化已在单分子水平上验证。
化学和生物缀合技术的最新发展允许对各种蛋白质聚合物进行工程设计。但是,大多数聚合过程难以控制。为了应对这一挑战,我们开发了一种酶促程序,可以使用严格的蛋白质连接酶OaAEP1(Oldenlandia affinis天冬酰胺基内肽酶1)和蛋白酶TEV(烟草蚀刻病毒)。我们首先证明单独使用OaAEP1来构建序列不受控制的泛素多蛋白并将共价偶联的蛋白共价固定在表面上。然后,我们从纯化的单体中构建了一种多金属蛋白,即rubredoxin。最后,我们显示了通过连接酶和蛋白酶的协同作用合成具有合理控制序列的蛋白质聚合物的可行性,这已通过使用基于原子力显微镜的单分子力光谱法(AFM-SMFS)展开蛋白质而得到验证。因此,这项研究为多蛋白工程和固定化提供了一种策略。






























 京公网安备 11010802027423号
京公网安备 11010802027423号