当前位置:
X-MOL 学术
›
ACS Appl. Energy Mater.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Chitin and Chitosan—Structurally Related Precursors of Dissimilar Hard Carbons for Na-Ion Battery
ACS Applied Energy Materials ( IF 5.4 ) Pub Date : 2019-06-24 00:00:00 , DOI: 10.1021/acsaem.9b00545 Joanna Conder 1, 2 , Cyril Vaulot 1, 2 , Cyril Marino 3 , Claire Villevieille 3 , Camélia Matei Ghimbeu 1, 2, 4
ACS Applied Energy Materials ( IF 5.4 ) Pub Date : 2019-06-24 00:00:00 , DOI: 10.1021/acsaem.9b00545 Joanna Conder 1, 2 , Cyril Vaulot 1, 2 , Cyril Marino 3 , Claire Villevieille 3 , Camélia Matei Ghimbeu 1, 2, 4
Affiliation
|
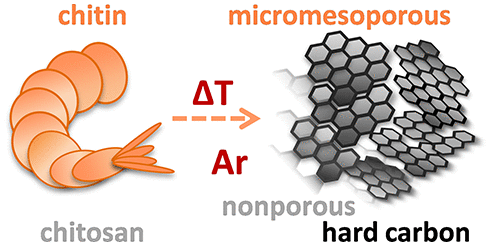
Hard carbons (HCs) prepared from renewable precursors are promising cost-effective electrode-material candidates for the application in Na-ion battery. Usually these materials are derived from cellulose. Here, however, we demonstrate that other polysaccharides, such as chitin and chitosan, can be as well up-and-coming parent materials of HCs. Despite structural similarities, thermal decomposition of these two biopolymers proceeds differently, contributing to the discrepancies in physicochemical properties of resulting HCs. Although chitin- and chitosan-derived HCs have comparable d-spacings and crystallite sizes, solid-state pyrolysis of the former biopolymer leads to micromesoporous material with significant specific surface area, while that of chitosan yields nonporous carbon. Despite that, both materials deliver similar initial specific charge of 280 mAh g–1 (at C/10 rate), and their electrochemical performance starts to diverge only upon longer cycling at higher rate. With time, inorganic contaminants present in chitosan-derived HC presumably delay the diffusion of Na ions to and within the electrode and slow the rate of electrochemical reactions, eventually triggering polarization buildup. Further optimization of the chitosan-derived HC through acid treatment enables unblocking some of the micropores and increasing the carbon content in this material, therefore enhancing its active surface area and suppressing continuous fading of the specific charge.
中文翻译:

甲壳素和壳聚糖—钠离子电池用不同硬碳的结构相关前体
由可再生前体制成的硬碳(HCs)是有希望在Na离子电池中应用的经济高效的电极材料候选产品。通常,这些材料衍生自纤维素。但是,在这里,我们证明了其他多糖,例如几丁质和壳聚糖,也可能是HC的新兴母体材料。尽管结构相似,但这两种生物聚合物的热分解过程却有所不同,从而导致了所得HC的理化特性差异。尽管几丁质和壳聚糖衍生的HC具有可比的d-间距和微晶尺寸,前一种生物聚合物的固态热解产生具有明显比表面积的微介孔材料,而壳聚糖则产生无孔碳。尽管如此,两种材料仍提供类似的280 mAh g –1的初始比电荷。(以C / 10的速率),它们的电化学性能只有在以更高的速率进行更长的循环后才开始发散。随着时间的流逝,壳聚糖衍生的HC中存在的无机污染物可能会延迟Na离子向电极内部和电极内部的扩散,并减慢电化学反应的速度,最终触发极化的建立。通过酸处理对壳聚糖衍生的HC进行进一步优化,可以解开一些微孔并增加该材料中的碳含量,因此提高了其活性表面积并抑制了比电荷的连续褪色。
更新日期:2019-06-24
中文翻译:

甲壳素和壳聚糖—钠离子电池用不同硬碳的结构相关前体
由可再生前体制成的硬碳(HCs)是有希望在Na离子电池中应用的经济高效的电极材料候选产品。通常,这些材料衍生自纤维素。但是,在这里,我们证明了其他多糖,例如几丁质和壳聚糖,也可能是HC的新兴母体材料。尽管结构相似,但这两种生物聚合物的热分解过程却有所不同,从而导致了所得HC的理化特性差异。尽管几丁质和壳聚糖衍生的HC具有可比的d-间距和微晶尺寸,前一种生物聚合物的固态热解产生具有明显比表面积的微介孔材料,而壳聚糖则产生无孔碳。尽管如此,两种材料仍提供类似的280 mAh g –1的初始比电荷。(以C / 10的速率),它们的电化学性能只有在以更高的速率进行更长的循环后才开始发散。随着时间的流逝,壳聚糖衍生的HC中存在的无机污染物可能会延迟Na离子向电极内部和电极内部的扩散,并减慢电化学反应的速度,最终触发极化的建立。通过酸处理对壳聚糖衍生的HC进行进一步优化,可以解开一些微孔并增加该材料中的碳含量,因此提高了其活性表面积并抑制了比电荷的连续褪色。

































 京公网安备 11010802027423号
京公网安备 11010802027423号