当前位置:
X-MOL 学术
›
Org. Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Direct α-Imination of N-Acyl Pyrazoles with Nitrosoarenes
Organic Letters ( IF 4.9 ) Pub Date : 2019-06-21 00:00:00 , DOI: 10.1021/acs.orglett.9b01913 Chiara Volpe 1 , Sara Meninno 1 , Giulia Mirra 1 , Jacob Overgaard 2 , Amedeo Capobianco 1 , Alessandra Lattanzi 1
Organic Letters ( IF 4.9 ) Pub Date : 2019-06-21 00:00:00 , DOI: 10.1021/acs.orglett.9b01913 Chiara Volpe 1 , Sara Meninno 1 , Giulia Mirra 1 , Jacob Overgaard 2 , Amedeo Capobianco 1 , Alessandra Lattanzi 1
Affiliation
|
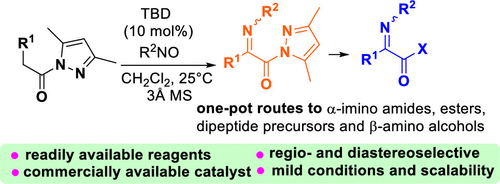
Unprecedented α-imino N-acyl pyrazoles were efficiently and selectively prepared through the 1,5,7-triazabicyclo[4.4.0]dec-5-ene (TBD)-catalyzed reaction of nitrosoarenes with N-acyl pyrazoles via an N-nitroso aldol reaction/dehydration sequence. The α-imino acyl pyrazoles were demonstrated to be new versatile intermediates for practical one-pot syntheses of α-imino amides, dipeptide precursors, esters, and β-amino alcohols. The synthetic method competes with known protocols in terms of ready availability of the reagents and catalyst, mild and catalytic reaction conditions, gram-scale applicability, and scope of the α-imino acid derivatives achievable.
中文翻译:

N-酰基吡唑与亚硝基芳烃的直接α-氨基化
通过1,5,7-三氮杂双环[4.4.0]癸-5-烯(TBD)催化的亚硝基芳烃与N-酰基吡唑的反应,通过N-亚硝基,有效地,选择性地制备了前所未有的α-亚氨基N-酰基吡唑羟醛反应/脱水顺序。已证明α-亚氨基酰基吡唑是α-亚氨基酰胺,二肽前体,酯和β-氨基醇的一锅合成的新型通用中间体。在试剂和催化剂的容易获得,温和的和催化的反应条件,克规模的适用性以及可获得的α-亚氨基酸衍生物的范围方面,该合成方法与已知的方案竞争。
更新日期:2019-06-21
中文翻译:

N-酰基吡唑与亚硝基芳烃的直接α-氨基化
通过1,5,7-三氮杂双环[4.4.0]癸-5-烯(TBD)催化的亚硝基芳烃与N-酰基吡唑的反应,通过N-亚硝基,有效地,选择性地制备了前所未有的α-亚氨基N-酰基吡唑羟醛反应/脱水顺序。已证明α-亚氨基酰基吡唑是α-亚氨基酰胺,二肽前体,酯和β-氨基醇的一锅合成的新型通用中间体。在试剂和催化剂的容易获得,温和的和催化的反应条件,克规模的适用性以及可获得的α-亚氨基酸衍生物的范围方面,该合成方法与已知的方案竞争。






























 京公网安备 11010802027423号
京公网安备 11010802027423号