当前位置:
X-MOL 学术
›
Environ. Sci. Technol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Decreases in Iron Oxide Reducibility during Microbial Reductive Dissolution and Transformation of Ferrihydrite
Environmental Science & Technology ( IF 10.8 ) Pub Date : 2019-07-24 , DOI: 10.1021/acs.est.9b01299 Meret Aeppli 1, 2 , Sanja Vranic 1 , Ralf Kaegi 2 , Ruben Kretzschmar 1 , Ashley R. Brown 2 , Andreas Voegelin 2 , Thomas B. Hofstetter 1, 2 , Michael Sander 1
Environmental Science & Technology ( IF 10.8 ) Pub Date : 2019-07-24 , DOI: 10.1021/acs.est.9b01299 Meret Aeppli 1, 2 , Sanja Vranic 1 , Ralf Kaegi 2 , Ruben Kretzschmar 1 , Ashley R. Brown 2 , Andreas Voegelin 2 , Thomas B. Hofstetter 1, 2 , Michael Sander 1
Affiliation
|
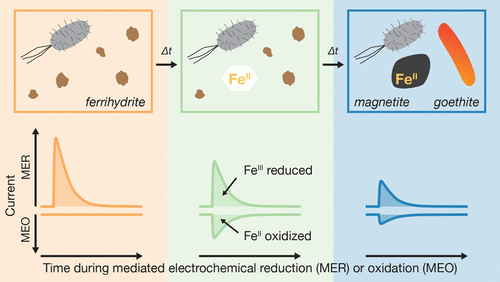
Ferrous iron formed during microbial ferric iron reduction induces phase transformations of poorly crystalline into more crystalline and thermodynamically more stable iron (oxyhydr)oxides. Yet, characterizing the resulting decreases in the reactivity of the remaining oxide ferric iron toward reduction (i.e., its reducibility) has been challenging. Here, we used the reduction of six-line ferrihydrite by Shewanella oneidensis MR-1 as a model system to demonstrate that mediated electrochemical reduction (MER) allows directly following decreases in oxide ferric iron reducibility during the transformation of ferrihydrite into goethite and magnetite which we characterized by X-ray diffraction analysis and transmission electron microscopy imaging. Ferrihydrite was fully reducible in MER at both pHMER of 5.0 and 7.5. Decreases in iron oxide reducibility associated with ferrihydrite transformation into magnetite were accessible at both pHMER because the formed magnetite was not reducible under either of these conditions. Conversely, decreases in iron oxide reducibility associated with goethite formation were apparent only at the highest tested pHMER of 7.5 and thus the thermodynamically least favorable conditions for iron oxide reductive dissolution. The unique capability to adjust the thermodynamic boundary conditions in MER to the specific reducibilities of individual iron (oxyhydr)oxides makes this electrochemical approach broadly applicable for studying changes in iron oxide reducibility in heterogeneous environmental samples such as soils and sediments.
中文翻译:

微生物还原溶解和水铁矿转化过程中氧化铁还原能力的降低
在微生物三价铁还原过程中形成的亚铁诱导了结晶度差的相转变为结晶度更高,热力学上更稳定的氧化铁(羟基氧化物)。然而,表征残留的氧化铁铁对还原的反应性(即其还原性)降低的结果是具有挑战性的。在这里,我们使用Shewanella oneidensis MR-1还原六线铁水合物作为模型系统,以证明介导的电化学还原(MER)可以在铁水合物转变为针铁矿和磁铁矿的过程中直接跟随氧化物三价铁还原性的降低。通过X射线衍射分析和透射电子显微镜成像进行表征。在两个pH MER下,水铁矿均可在MER中完全还原分别为5.0和7.5。在两个pH MER下,铁水铁矿转变为磁铁矿相关的氧化铁还原性均可以降低,因为形成的磁铁矿在任何一种条件下均不可还原。相反,与针铁矿形成相关的氧化铁还原性的降低仅在最高测试pH MER为7.5时才明显,因此在热力学上不利于氧化铁还原性溶解的条件也很明显。能够将MER中的热力学边界条件调整为单个氧化铁(羟基氧化物)的特定还原度的独特功能,使这种电化学方法广泛适用于研究非均质环境样品(例如土壤和沉积物)中氧化铁还原性的变化。
更新日期:2019-07-25
中文翻译:

微生物还原溶解和水铁矿转化过程中氧化铁还原能力的降低
在微生物三价铁还原过程中形成的亚铁诱导了结晶度差的相转变为结晶度更高,热力学上更稳定的氧化铁(羟基氧化物)。然而,表征残留的氧化铁铁对还原的反应性(即其还原性)降低的结果是具有挑战性的。在这里,我们使用Shewanella oneidensis MR-1还原六线铁水合物作为模型系统,以证明介导的电化学还原(MER)可以在铁水合物转变为针铁矿和磁铁矿的过程中直接跟随氧化物三价铁还原性的降低。通过X射线衍射分析和透射电子显微镜成像进行表征。在两个pH MER下,水铁矿均可在MER中完全还原分别为5.0和7.5。在两个pH MER下,铁水铁矿转变为磁铁矿相关的氧化铁还原性均可以降低,因为形成的磁铁矿在任何一种条件下均不可还原。相反,与针铁矿形成相关的氧化铁还原性的降低仅在最高测试pH MER为7.5时才明显,因此在热力学上不利于氧化铁还原性溶解的条件也很明显。能够将MER中的热力学边界条件调整为单个氧化铁(羟基氧化物)的特定还原度的独特功能,使这种电化学方法广泛适用于研究非均质环境样品(例如土壤和沉积物)中氧化铁还原性的变化。















































 京公网安备 11010802027423号
京公网安备 11010802027423号