当前位置:
X-MOL 学术
›
Adv. Funct. Mater.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Targeting of CXCR1 on Osteosarcoma Circulating Tumor Cells and Precise Treatment via Cisplatin Nanodelivery
Advanced Functional Materials ( IF 18.5 ) Pub Date : 2019-06-20 , DOI: 10.1002/adfm.201902246
Xiu‐guo Han 1 , Sheng‐bing Yang 1 , Hui‐min Mo 2 , Min‐qi Wang 1 , Feng Zhou 1 , Han‐jun Li 1 , Han Qiao 1 , Jing‐tian Mei 1 , Yong‐jie Wang 3 , Ya‐wen Cheng 4 , Xu‐qiang Liu 5 , Lin Du 6 , Yang Dong 3 , Ting‐ting Tang 1
Advanced Functional Materials ( IF 18.5 ) Pub Date : 2019-06-20 , DOI: 10.1002/adfm.201902246
Xiu‐guo Han 1 , Sheng‐bing Yang 1 , Hui‐min Mo 2 , Min‐qi Wang 1 , Feng Zhou 1 , Han‐jun Li 1 , Han Qiao 1 , Jing‐tian Mei 1 , Yong‐jie Wang 3 , Ya‐wen Cheng 4 , Xu‐qiang Liu 5 , Lin Du 6 , Yang Dong 3 , Ting‐ting Tang 1
Affiliation
|
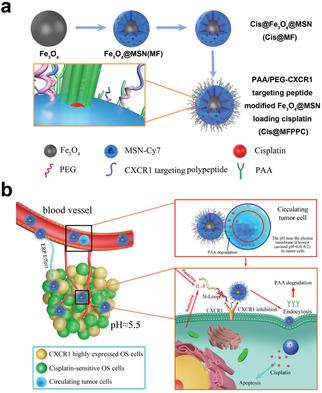
Metastasis and chemotherapy resistance are the key factors affecting the effectiveness of osteosarcoma (OS) treatments. CXCR1 overexpression is found to be closely related to chemotherapy resistance and anoikis resistance in OS cell subtypes with high metastasis potential. Further study demonstrates that CXCR1 is highly expressed on circulating tumor cell (CTC)‐derived cells with cancer stem cell characteristics. Then, a CXCR1 targeting peptide is designed and synthesized to competitively inhibit the IL‐8/CXCR1 pathway and to improve the cisplatin sensitivity of CTCs. Fluorescence‐labeled magnetic nanoparticles (NPs) with pH‐responsive cisplatin release are fabricated and linked with the CXCR1 targeting peptide (Cis@MFPPC). Results demonstrate that CTC survival could be inhibited effectively by the targeting nanoparticles in vivo. Cis@MFPPC can also inhibit OS growth and pulmonary metastasis in an orthotopic model and patient‐derived tumor xenograft model. This study verifies the clinical significance of CXCR1 as a therapeutic target and provides a drug delivery NP system for precise treatment of OS.
中文翻译:

CXCR1靶向骨肉瘤循环肿瘤细胞并通过顺铂纳米递送进行精确治疗
转移和化疗耐药性是影响骨肉瘤(OS)治疗有效性的关键因素。在具有高转移潜能的OS细胞亚型中,发现CXCR1的过表达与化疗耐药性和对Anoikis的耐药性密切相关。进一步的研究表明,CXCR1在具有癌症干细胞特征的循环肿瘤细胞(CTC)衍生的细胞上高表达。然后,设计并合成了一种CXCR1靶向肽,以竞争性抑制IL-8 / CXCR1途径并提高CTC的顺铂敏感性。可以制造具有pH响应的顺铂释放的荧光标记磁性纳米颗粒(NP),并与CXCR1靶向肽(Cis @ MFPPC)连接。结果表明,在体内靶向纳米粒子可以有效地抑制四氯化碳的存活。在原位模型和患者源性肿瘤异种移植模型中,Cis @ MFPPC还可以抑制OS生长和肺转移。这项研究验证了CXCR1作为治疗靶标的临床意义,并提供了用于OS精确治疗的药物递送NP系统。
更新日期:2019-06-20
中文翻译:

CXCR1靶向骨肉瘤循环肿瘤细胞并通过顺铂纳米递送进行精确治疗
转移和化疗耐药性是影响骨肉瘤(OS)治疗有效性的关键因素。在具有高转移潜能的OS细胞亚型中,发现CXCR1的过表达与化疗耐药性和对Anoikis的耐药性密切相关。进一步的研究表明,CXCR1在具有癌症干细胞特征的循环肿瘤细胞(CTC)衍生的细胞上高表达。然后,设计并合成了一种CXCR1靶向肽,以竞争性抑制IL-8 / CXCR1途径并提高CTC的顺铂敏感性。可以制造具有pH响应的顺铂释放的荧光标记磁性纳米颗粒(NP),并与CXCR1靶向肽(Cis @ MFPPC)连接。结果表明,在体内靶向纳米粒子可以有效地抑制四氯化碳的存活。在原位模型和患者源性肿瘤异种移植模型中,Cis @ MFPPC还可以抑制OS生长和肺转移。这项研究验证了CXCR1作为治疗靶标的临床意义,并提供了用于OS精确治疗的药物递送NP系统。

































 京公网安备 11010802027423号
京公网安备 11010802027423号