当前位置:
X-MOL 学术
›
J. Phys. Chem. C
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Water (Non-)Interaction with MoO3.
The Journal of Physical Chemistry C ( IF 3.3 ) Pub Date : 2019-07-01 , DOI: 10.1021/acs.jpcc.9b03822 Ashley R Head 1 , Chiara Gattinoni 2 , Lena Trotochaud 3 , Yi Yu 3, 4 , Osman Karslıoğlu 3 , Sven Pletincx 3, 5 , Bryan Eichhorn 4 , Hendrik Bluhm 3, 3, 6
The Journal of Physical Chemistry C ( IF 3.3 ) Pub Date : 2019-07-01 , DOI: 10.1021/acs.jpcc.9b03822 Ashley R Head 1 , Chiara Gattinoni 2 , Lena Trotochaud 3 , Yi Yu 3, 4 , Osman Karslıoğlu 3 , Sven Pletincx 3, 5 , Bryan Eichhorn 4 , Hendrik Bluhm 3, 3, 6
Affiliation
|
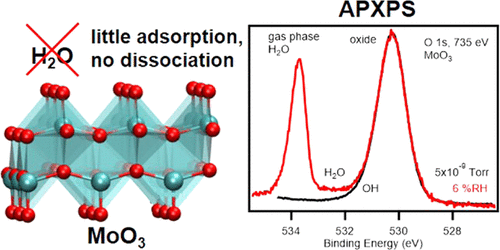
Molybdenum(VI) oxide (MoO3) is used in a number of technical processes such as gas filtration and heterogeneous catalysis. In these applications, the adsorption and dissociation of water on the surface can influence the chemistry of MoO3 and thus the course of heterogeneous reactions. We use ambient pressure X-ray photoelectron spectroscopy to study the interaction of water with a stoichiometric MoO3 surface and a MoO3 surface that features oxygen defects and hydroxyl groups. The experimental results are supported by density functional theory calculations. We show that on a stoichiometric MoO3(010) surface, where Mo sites are unavailable, water adsorption is strongly disfavored. However, the introduction of surface species, which can interact with the lone pairs on the water O atom, e.g., Mo5+ atoms or surface OH groups, promotes water adsorption. Dissociation of water is favored at unsaturated Mo sites, i.e., at oxygen vacancies, while water adsorbs molecularly at hydroxyl sites.
中文翻译:

水与 MoO3 的(非)相互作用。
氧化钼(VI) (MoO 3 ) 用于许多技术过程,例如气体过滤和多相催化。在这些应用中,水在表面的吸附和解离可以影响MoO 3的化学性质,从而影响多相反应的过程。我们使用常压 X 射线光电子能谱研究水与化学计量 MoO 3表面以及具有氧缺陷和羟基的 MoO 3表面的相互作用。实验结果得到了密度泛函理论计算的支持。我们表明,在化学计量的 MoO 3 (010) 表面上,Mo 位点不可用,因此强烈不利于水吸附。然而,引入可与水O原子上的孤对电子相互作用的表面物质,例如Mo 5+原子或表面OH基团,促进水吸附。水在不饱和Mo位点(即氧空位)处有利于解离,而水在羟基位点处进行分子吸附。
更新日期:2019-07-01
中文翻译:

水与 MoO3 的(非)相互作用。
氧化钼(VI) (MoO 3 ) 用于许多技术过程,例如气体过滤和多相催化。在这些应用中,水在表面的吸附和解离可以影响MoO 3的化学性质,从而影响多相反应的过程。我们使用常压 X 射线光电子能谱研究水与化学计量 MoO 3表面以及具有氧缺陷和羟基的 MoO 3表面的相互作用。实验结果得到了密度泛函理论计算的支持。我们表明,在化学计量的 MoO 3 (010) 表面上,Mo 位点不可用,因此强烈不利于水吸附。然而,引入可与水O原子上的孤对电子相互作用的表面物质,例如Mo 5+原子或表面OH基团,促进水吸附。水在不饱和Mo位点(即氧空位)处有利于解离,而水在羟基位点处进行分子吸附。

































 京公网安备 11010802027423号
京公网安备 11010802027423号