当前位置:
X-MOL 学术
›
Sci. Total Environ.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Preparation of core-shell structure Fe3O4@C@MnO2 nanoparticles for efficient elimination of U(VI) and Eu(III) ions
Science of the Total Environment ( IF 8.2 ) Pub Date : 2019-06-19 , DOI: 10.1016/j.scitotenv.2019.06.292 Shuhui Dai , Ning Wang , Chenjia Qi , Xiangxue Wang , Yan Ma , Lu Yang , Xiaoyan Liu , Qiang Huang , Changming Nie , Baowei Hu , Xiangke Wang
Science of the Total Environment ( IF 8.2 ) Pub Date : 2019-06-19 , DOI: 10.1016/j.scitotenv.2019.06.292 Shuhui Dai , Ning Wang , Chenjia Qi , Xiangxue Wang , Yan Ma , Lu Yang , Xiaoyan Liu , Qiang Huang , Changming Nie , Baowei Hu , Xiangke Wang
|
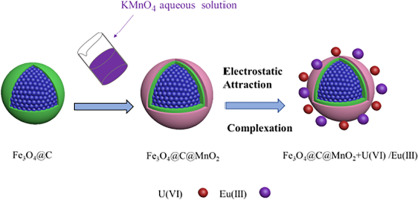
Radionuclide contamination has become an urgent problem with the development of nuclear power plants. Herein, chemical-decorated core-shell magnetic manganese dioxide (denoted as Fe3 O4 @C@MnO2 ) composites were synthesized via transforming KMnO4 to MnO2 on the carbon-covered magnetite (Fe3 O4 @C) microsphere surface. It was employed to remove U(VI) and Eu(III) ions from aqueous solution under various conditions. The kinetic adsorption data were well simulated by the pseudo-second-order model and adsorption isotherms were fitted well by Langmuir model. Moreover, the maximum uptake capacities were up to 77.71 mg/g for U(VI) and 51.01 mg/g for Eu(III) at pH = 5.0 and T = 298 K. Adsorption behavior was strongly related to pH values but weakly affected by ionic strength, implying that the interaction of U(VI)/Eu(III) with Fe3 O4 @C@MnO2 was mainly dominated by inner-sphere surface complexation. XPS analysis illustrated that the interaction of Eu(III)/U(VI) with Fe3 O4 @C@MnO2 was associated with the strong metal bonds (Mn3 O4 @C@MnO2 in eliminating Eu(III)/U(VI) ions from aqueous solutions, which was of great significance in the future application in radionuclides' pollution treatment.
中文翻译:

制备核壳结构Fe3O4@C@MnO2纳米颗粒以有效消除 U(VI) 和 Eu(III) 离子
放射性核素污染已成为核电站发展的亟待解决的问题。在此,通过在碳覆盖的磁铁矿 (Fe3O4@C) 微球表面将 KMnO4 转化为 MnO2 合成了化学修饰的核壳磁性二氧化锰 (表示为 Fe3O4@C@MnO2) 复合材料。它用于在各种条件下从水溶液中去除 U(VI) 和 Eu(III) 离子。准二级模型对动力学吸附数据进行了较好的模拟,Langmuir 模型对吸附等温线进行了较好的拟合。此外,在 pH = 5.0 和 T = 298 K 时,U(VI) 和 Eu(III) 的最大吸收量高达 77.71 mg/g,Eu(III) 的最大吸收量高达 51.01 mg/g。吸附行为与 pH 值密切相关,但受离子强度的影响较弱,表明 U(VI)/Eu(III) 与 Fe3O4@C@MnO2 的相互作用主要以球内表面络合为主。XPS 分析表明,Eu(III)/U(VI) 与 Fe3O4@C@MnO2 的相互作用与强金属键 (MnO)、金属上的羟基键 (Mn-OH) 和羧基 (-COOH) 通过表面络合相关,zeta 电位结果表明吸附过程受静电吸引控制。本研究突出了 Fe3O4@C@MnO2 在去除水溶液中 Eu(III)/U(VI) 离子方面的出色性能,这对未来放射性核素污染处理的应用具有重要意义。
更新日期:2019-06-19
中文翻译:

制备核壳结构Fe3O4@C@MnO2纳米颗粒以有效消除 U(VI) 和 Eu(III) 离子
放射性核素污染已成为核电站发展的亟待解决的问题。在此,通过在碳覆盖的磁铁矿 (Fe3O4@C) 微球表面将 KMnO4 转化为 MnO2 合成了化学修饰的核壳磁性二氧化锰 (表示为 Fe3O4@C@MnO2) 复合材料。它用于在各种条件下从水溶液中去除 U(VI) 和 Eu(III) 离子。准二级模型对动力学吸附数据进行了较好的模拟,Langmuir 模型对吸附等温线进行了较好的拟合。此外,在 pH = 5.0 和 T = 298 K 时,U(VI) 和 Eu(III) 的最大吸收量高达 77.71 mg/g,Eu(III) 的最大吸收量高达 51.01 mg/g。吸附行为与 pH 值密切相关,但受离子强度的影响较弱,表明 U(VI)/Eu(III) 与 Fe3O4@C@MnO2 的相互作用主要以球内表面络合为主。XPS 分析表明,Eu(III)/U(VI) 与 Fe3O4@C@MnO2 的相互作用与强金属键 (MnO)、金属上的羟基键 (Mn-OH) 和羧基 (-COOH) 通过表面络合相关,zeta 电位结果表明吸附过程受静电吸引控制。本研究突出了 Fe3O4@C@MnO2 在去除水溶液中 Eu(III)/U(VI) 离子方面的出色性能,这对未来放射性核素污染处理的应用具有重要意义。

































 京公网安备 11010802027423号
京公网安备 11010802027423号