Nature Communications ( IF 14.7 ) Pub Date : 2019-06-20 , DOI: 10.1038/s41467-019-10641-y Kohsuke Ohmatsu 1 , Tsubasa Nakashima 1 , Makoto Sato 1 , Takashi Ooi 1, 2
|
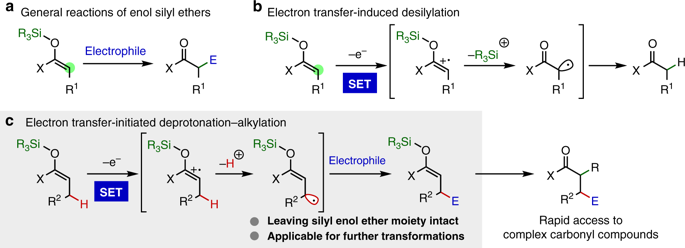
Strategies for altering the reaction pathway of reactive intermediates are of significant importance in diversifying organic synthesis. Enol silyl ethers, versatile enolate equivalents, are known to undergo one-electron oxidation to generate the radical cations that spontaneously form electrophilic α-carbonyl radicals via elimination of the silyl groups. Here, we demonstrate that close scrutiny of the property of the radical cations as strong C–H acids enables the identification of a catalyst system consisting of an iridium-based photosensitizer and 2,4,6-collidine for the generation of nucleophilic allylic radicals from enol silyl ethers through one-electron oxidation-deprotonation sequence under light irradiation without the desilylation of the radical cation intermediates. The resultant allylic radicals engage in the addition to electron-deficient olefins, establishing the selective allylic C-H alkylation of enol silyl ethers. This strategy is broadly applicable, and the alkylated enol silyl ethers can be transformed into highly functionalized carbonyl compounds by exploiting their common polar reactivity.
中文翻译:

通过光氧化还原布朗斯台德碱杂化催化实现烯醇甲硅烷基醚的直接烯丙基CH烷基化。
改变反应性中间体的反应途径的策略在使有机合成多样化方面具有重要意义。已知烯醇甲硅烷基醚,通用的烯醇酸酯等效物,经历单电子氧化以产生自由基阳离子,该自由基阳离子通过消除甲硅烷基基团自发形成亲电性α-羰基自由基。在这里,我们证明了对作为强C–H酸的自由基阳离子的性质进行的仔细审查,使得能够识别由铱基光敏剂和2,4,6-可力丁组成的催化剂体系,从而从中生成亲核烯丙基基团。烯醇甲硅烷基醚在光照射下通过单电子氧化-去质子化序列而没有自由基阳离子中间体的甲硅烷基化。所得的烯丙基自由基参与电子不足的烯烃的加成反应,从而建立了烯醇甲硅烷基醚的选择性烯丙基CH烷基化。该策略广泛适用,并且烷基化的烯醇甲硅烷基醚可以通过利用它们的常见极性反应性而转变成高度官能化的羰基化合物。

































 京公网安备 11010802027423号
京公网安备 11010802027423号