当前位置:
X-MOL 学术
›
Org. Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
TMSCl-Catalyzed Electrophilic Thiocyano Oxyfunctionalization of Alkenes Using N-Thiocyano-dibenzenesulfonimide
Organic Letters ( IF 4.9 ) Pub Date : 2019-06-20 00:00:00 , DOI: 10.1021/acs.orglett.9b01706 Ai-Hui Ye 1 , Ye Zhang 1 , Yu-Yang Xie 1 , Hui-Yun Luo 1 , Jia-Wei Dong 1 , Xiao-Dong Liu 1 , Xu-Feng Song 1 , Tongmei Ding 1 , Zhi-Min Chen 1
Organic Letters ( IF 4.9 ) Pub Date : 2019-06-20 00:00:00 , DOI: 10.1021/acs.orglett.9b01706 Ai-Hui Ye 1 , Ye Zhang 1 , Yu-Yang Xie 1 , Hui-Yun Luo 1 , Jia-Wei Dong 1 , Xiao-Dong Liu 1 , Xu-Feng Song 1 , Tongmei Ding 1 , Zhi-Min Chen 1
Affiliation
|
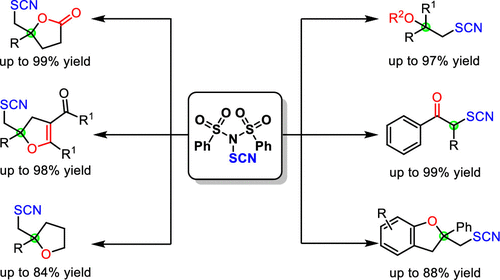
Numerous electrophilic thiocyano oxyfunctionalization reactions of alkenes have been achieved using N-thiocyano-dibenzenesulfonimide, which is a new electrophilic thiocyanation reagent and could be easily prepared in two steps from dibenzenesulfonimide. This approach provides efficient, simple, and modular methods for the formation of SCN-containing heterocycles such as lactones, tetrahydrofurans, dihydrofurans, and dihydrobenzofurans in moderate to excellent yields. Meanwhile, diverse oxa-quaternary centers were rapidly constructed. Additionally, this protocol is free of transition metals and features broad substrate toleraance and mild reaction conditions.
中文翻译:

N-硫代氰基-二苯磺酰亚胺对TMSC1催化的烯烃亲电硫代氰基氧基官能化
使用N-硫氰基-二苯磺酰亚胺是一种新型的亲电子硫氰化试剂,可以很容易地从二苯磺酰亚胺分两步制备,实现了烯烃的许多亲电子硫氰基氧基官能化反应。该方法提供了有效,简单和模块化的方法,以中等到极好的收率形成含SCN的杂环,如内酯,四氢呋喃,二氢呋喃和二氢苯并呋喃。同时,迅速建立了各种各样的氧杂-季铵盐中心。此外,该方案不含过渡金属,并具有宽广的底物耐受性和温和的反应条件。
更新日期:2019-06-20
中文翻译:

N-硫代氰基-二苯磺酰亚胺对TMSC1催化的烯烃亲电硫代氰基氧基官能化
使用N-硫氰基-二苯磺酰亚胺是一种新型的亲电子硫氰化试剂,可以很容易地从二苯磺酰亚胺分两步制备,实现了烯烃的许多亲电子硫氰基氧基官能化反应。该方法提供了有效,简单和模块化的方法,以中等到极好的收率形成含SCN的杂环,如内酯,四氢呋喃,二氢呋喃和二氢苯并呋喃。同时,迅速建立了各种各样的氧杂-季铵盐中心。此外,该方案不含过渡金属,并具有宽广的底物耐受性和温和的反应条件。































 京公网安备 11010802027423号
京公网安备 11010802027423号